Physical principles of radiation detection in sample counters
Published July 2022
•
Copyright © IOP Publishing Ltd 2022
Pages 1-1 to 1-11
You need an eReader or compatible software to experience the benefits of the ePub3 file format.
Download complete PDF book, the ePub book or the Kindle book
Abstract
Chapter 1 outlines the physics of radiation detection, focusing on the properties of sodium iodide detectors. The chapter closes with brief descriptions of liquid scintillators and solid state detectors.
1.1. Introduction
There are currently many clinical applications, both routine and research, that involve assessing the radioactivity in samples of body fluids. These include radioimmunoassay, body volume measurements, and tracer kinetic studies of absorption and clearance.
Most sample counters detect radiation by scintillation spectrometry, in which gamma-ray, alpha-, or beta-particle energy is converted into light photons by a detector. The first use of this process was by Crookes and Regener (Birks 1964), who in 1908 detected alpha particle interactions in zinc sulphide by visual observation of the light emitted. The invention of the photomultiplier tube (PMT), which enabled the light energy to be converted into an electrical pulse, greatly enhanced the ability of scintillators to count particle interactions (Blau and Dreyfus 1945). At around the same time, new scintillators were discovered, including thallium-activated sodium iodide (NaI(Tl)) (Hofstadter 1949) and organic liquids (Kallmann 1950); these soon became established as valuable for counting gamma and beta samples, respectively (Anger 1951, Raben and Bloembergen 1951). Alternative scintillator materials include thallium-activated caesium iodide (CsI(Tl)), which is routinely used in gamma probe systems, as described in chapter 5.
More recently semiconductor detectors, such as cadmium telluride (CdTe) and cadmium zinc telluride (CZT), have been used in intraoperative gamma probes. Their compact size and excellent energy resolution (Zanzonico and Heller 2000) makes them suitable for intraoperative measurements of radioactivity to help pinpoint the locations of small regions of clinical interest (such as lymph nodes) in vivo and facilitate minimally invasive node resection. Automatic blood sampling systems that use a pump and online sample counters have become available, facilitating real-time assay of radioactivity in blood for tracer kinetic studies. These are mostly used with short-lived radionuclides for PET pharmacokinetic studies.
The counting of gamma or x-ray emitters is accomplished by external detection, as their photons readily pass through the sample material and the vial wall. Detectors made of solid materials with high atomic numbers are preferred, due to their high counting efficiency. They should ideally also be able to measure the energy loss of events to allow different emissions to be separately identified. Inorganic scintillators fit these requirements well, among which NaI(Tl) is the best suited for gamma sample counters. Semiconductor detectors have superior energy resolution when compared to inorganic scintillators, but generally have significantly lower counting efficiency.
The counting of beta emitters is more difficult, as the energy of the particles is efficiently absorbed in the sample material and the vial wall. Liquid scintillation counting is the principal method used for assaying beta-emitting samples, as the scintillants can be mixed with the sample. The beta particles are then detected in the scintillator before they lose any energy in intervening attenuating material, so providing a 4π solid-angle counting geometry. It is also possible to use liquid scintillators for alpha particle and gamma-ray detection. Liquid scintillation counting is described in chapter 6.
1.2. Light-emitting processes
Scintillators detect radiation by the process of luminescence, which is the emission of light from a material in which electronic excitations have occurred. These excitations may arise from the absorption of nuclear radiation, but can also be caused by such processes as light absorption, chemical reactions, thermal heating, and electrical discharge.
1.3. Sodium iodide detectors
The detector used in most modern gamma counters is a single crystal of NaI(Tl). This has been the detector of choice for gamma counting since its invention in the late 1940s (Hine 1967). Pure sodium iodide scintillates only at low temperatures; however, the incorporation of thallium impurity atoms at a concentration of about 0.2% produces crystal imperfections, known as luminescence centres, which can be excited by ionising radiation at room temperature (Birks 1964).
The selection of NaI(Tl) is based on several physical properties:
- A density of 3.67 g cm−3 and an effective atomic number of 50 make it an efficient absorber of low- and medium-energy gamma rays up to ≈300 keV.
- It provides a signal which is proportional to the energy lost in the crystal and can therefore be used for energy-selective counting.
- It has a relatively high yield of ≈40 photons per keV of absorbed energy at room temperature, giving an energy resolution which is adequate for most applications of sample counters.
- It has a decay time of 0.23 μs. This enables reasonably high count rates to be achieved without significant dead-time loss, although this is a limitation in some applications of the detector.
- It is transparent and therefore large detectors can be constructed without significantly reducing the amount of light detected.
However, it does have some disadvantages which have to be considered in the design and use of detectors. The crystals are quite fragile and may be fractured by mechanical pressure or by temperature change (see section 4.6.5). NaI(Tl) is also hygroscopic, and exposure to the atmosphere produces yellow discoloration which attenuates the light output. For these reasons the crystal is sealed in an aluminium case. Even a sealed crystal eventually undergoes yellowing and this ultimately limits the lifetime of a detector to around 10–15 years. The environment of the detectors also has to be carefully controlled, in particular to avoid rapid temperature change.
1.3.1. Primary interaction processes
Gamma rays passing through the crystal are initially attenuated by photoelectric, Compton, or pair-production interactions.
The photoelectric effect is an event in which the gamma ray transfers all its energy to an inner orbital electron of an atom, resulting in the ejection of a bound electron. The electron is slowed down in the sodium iodide crystal by collisions with other electrons and becomes part of the general electron population. Some of this kinetic energy is transformed into light, as described in more detail below.
Compton scattering occurs when a gamma ray interacts with an outer orbital electron, which is regarded as a free electron. The photon is scattered with reduced energy and its energy loss is transferred to the electron as kinetic energy.
Pair production is an event which occurs in the high electric field close to the nucleus of an atom. The gamma-ray energy is converted to mass and creates an electron–positron pair. This can only occur for gamma-ray photons with a minimum energy of 1.02 MeV, the energy equivalent of the rest mass of the electron–positron pair. Any energy in excess of 1.02 MeV is partitioned as kinetic energy between the two particles. Both the electron and positron are slowed down, rapidly losing their kinetic energy in collisions with absorber electrons. The positron then undergoes annihilation with one of the electrons. The mass of the two particles is converted into energy, producing two gamma photons, each with an energy of 511 keV which are emitted at approximately 180° to each other. In crystals of the typical size used in gamma counters, these annihilation photons have a reasonably high probability of escaping from the detector without interaction.
The relative importance of these absorption processes for various gamma-ray energies is shown figure 1.1, which is a plot of the respective absorption coefficients against gamma-ray energy for both NaI(Tl) and organic liquid scintillators (sections 1.3 and 1.4).
Figure 1.1. Energy dependence of the absorption processes for NaI(Tl) and organic liquid scintillator.
Download figure:
Standard image High-resolution image1.3.2. Secondary interaction processes
Each of the primary interaction processes produces an energetic secondary electron, which loses energy by exciting other electrons in the crystal from the ground state to the conduction band, creating electron–hole pairs. As these excited electrons return to the ground state, they give rise to photons in the wavelength range of 300–500 nm. This range is partly in the visible region of the electromagnetic spectrum and partly in the near-UV region. About 20–30 photons are produced per keV of energy loss. Most of the rest of the energy is dissipated as heat. The number of photons produced is proportional to the energy lost by the gamma ray in the crystal. The intensity of luminescence is temperature dependent, with an energy peak that increases in size with decreasing detector temperature. For NaI(Tl) the decay time of luminescence is 0.23 μs. The light photons are detected by a PMT which converts their energy to a pulse in an electrical circuit, the size of which is proportional to the light detected by the photocathode (section 2.2).
1.3.3. The pulse size spectrum
A spectrum of pulses is produced as a result of the different interaction processes undergone in the detector. The voltage values of the pulse heights are converted to energy losses in keV. A typical spectrum for 137Cs obtained from a 50 mm × 50 mm NaI(Tl) crystal is shown in figure 1.2, which illustrates some of the common features listed below. For further information on the pulse size spectrum, see chapter 10 of Physics in Nuclear Medicine (Cherry et al 2012).
- 1.Photopeak: two main processes contribute to the photopeak:
- (a)Photoelectric: the total energy of a gamma ray is absorbed in the scintillator by the initial photoelectric event, followed by absorption within the scintillator of any resulting scattered electrons and x-rays, giving rise to a full-energy peak (photopeak).
- (b)Compton: the total energy of a gamma ray is absorbed in the scintillator by a single or multiple Compton scattering events, followed by a photoelectric event. The probability of such absorption increases with crystal thickness.
- 2.Compton plateau: during Compton scatter events, when the scattered photon escapes the detector without further interaction, the energy deposited in the crystal is less than that of the photopeak and depends on the scatter angle. This process gives rise to the Compton continuum. The continuum includes a broad spectrum of pulse sizes, corresponding to the variable energy of the secondary electrons produced by the Compton scattering process. The upper limit of the secondary electron energy corresponds to a photon scattering angle of 180° and gives rise to a feature known as the Compton edge.
- 3.Barium x-ray peak: many gamma-emitting radionuclides have an alternative decay path by internal conversion which results in the emission of a characteristic x-ray from the daughter nucleus. In the case of 137Cs, this is a barium x-ray at 37 keV.
- 4.Backscatter peak: this occurs when the gamma rays are backscattered into the scintillator after undergoing a Compton scattering event in the surrounding materials. The energy of the backscatter peak is approximately equal to the energy of a 180° Compton-scattered photon and approaches a maximum of 0.25 MeV as the energy of the incident gamma ray increases. Lead peak: Photoelectric interaction with nearby materials, such as detector shielding, can produce characteristic K x-rays from the shielding material. In the case of lead shielding these K x-rays lie in the energy range of 72–88 keV (NIST 2020). However, these lead peaks are not always seen in practice (see figure 10.6).
- 5.X-ray escape peak: a characteristic x-ray may be produced following the photoelectric absorption corresponding to the K x-ray of iodine (28 keV). In most cases the x-ray is reabsorbed; however, if the photoelectric absorption occurs near the surface region, the x-ray can escape, which results in a decrease of the deposited energy. This x-ray escape peak is most likely to be observed with lower-energy gamma rays since, in this case, most events occur close to the surface of the crystal.
- 6.Summation peak (true coincidence): if radionuclides emit two gamma rays from the same disintegration, a coincidence or summation peak may occur. This peak corresponds to the sum of the gamma-ray energies and arises because of the simultaneous absorption of both gamma rays in the scintillator. Summation peaks are routinely used, for example, when counting 111In or 125I. These can additionally help to correct the counting efficiency, as described in section 6.8.
- 7.Summation peak (random coincidence): Summation peaks may also occur for radionuclides emitting monoenergetic gamma rays when there is a high count rate. When two gamma rays are emitted by different nuclei within the time resolution of the electronic detection system (random coincidence), they result in a single event with a summed energy. The probability of random coincidence increases as the count rate increases. This summation peak leads to loss of events for individual gamma-ray responses at high count rates. These can be corrected for, as described in section 2.5.2., although samples can ideally be diluted or left to decay before counting to reduce count losses due to random coincidences. Alternatively, some modern counters have a high-count-rate mode, which uses a robotic arm to lift the sample partly off the well to reduce counting efficiency and therefore random coincidences.
Figure 1.2. Gamma-ray spectrum of 137Cs in a sodium iodide (Tl) detector.
Download figure:
Standard image High-resolution image1.3.4. Energy resolution
When a NaI(Tl) detector is exposed to a monoenergetic beam of gamma rays, all photoelectric events (except for a few in which the iodine x-ray escapes) have the same energy loss in the detector. However, as can be seen from figure 1.2, the photopeak comprises of a spread of pulse sizes. This results from random variations in the various energy conversion steps in the detection process. The width of the peak is primarily determined by the statistical variation in the number of photoelectrons produced at the photocathode of the PMT. The energy resolution is usually measured using the full width at half maximum (FWHM) of the photopeak and is often expressed as a fraction of the photopeak energy. For 137Cs the energy resolution of the 662 keV peak is typically about 7% (i.e. the FWHM is ~45keV). As the resolution is statistically limited, it improves with increasing gamma-ray energy proportional to 1/E1/2. The relatively good energy resolution of NaI(Tl) detectors means that it is possible to count samples containing a mixture of more than one radionuclide by using dual energy window counting and making appropriate correction for crosstalk between channels (section 9.7).
1.3.5. Counting efficiency
The counting of a sample is achieved by setting an appropriate pulse size window, which is often centered around the photopeak of the spectrum of the radionuclide. The counting efficiency (E) is defined as:
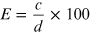
where c is the number of counts per second detected and d is the number of disintegrations per second in the sample. It depends on several factors, which can be described by the following equation:
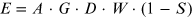
where A is the abundance of gamma photon production by the radionuclide, G is the geometric efficiency of photon detection, D is the intrinsic efficiency of the detector, W is the fraction of counts detected in the selected energy window, and S is the fraction of photons attenuated in the sample or vial wall.
The geometric efficiency is maximised by surrounding the sample as completely as possible with the detector. Fortunately, NaI(Tl) can be machined into a variety of shapes; those most commonly used are a well, first described by Anger (1951), and a diametric through hole (see figure 1.3), both of which give a geometric efficiency of almost 100 % with small volume samples. However, geometric efficiency is very dependent on the sample volume, particularly if activity extends near to or beyond the edge of the detector, and this must be taken into consideration when carrying out measurements (see section 4.6.13 for sample volume effects).
Figure 1.3. NaI(Tl) detectors: (a) well-type; (b) diametric through-hole type.
Download figure:
Standard image High-resolution imageThe size of the sample also influences the self-absorption fraction, S. This effect is more marked for low-energy emitters such as 125I (with energy peaks at 27 keV and 35.5 keV). For a particular application, the use of a constant sample volume and shape ensures that the geometric efficiency remains fixed. The intrinsic efficiency, D, is defined as the ratio of the number of pulses interacting in the detector to the number of gamma-ray photons which enter it. It increases with increasing crystal size and decreasing gamma-ray energy (figure 1.4(a)). The intrinsic efficiency value is close to unity for gamma-ray energies of less than 200 keV and medium-sized crystals.
Figure 1.4. The variation of (a) intrinsic efficiency and (b) photofraction with energy for well-shaped NaI(Tl) crystals of varying size.
Download figure:
Standard image High-resolution imageThe counting efficiency is also affected by the fraction of the detected spectrum which is used. It is common practice to eliminate rays scattered within the sample by counting only the photopeak, although in many applications this is not essential. Increasing the window width increases the relative contribution of background counts. If background contributions are substantial then a photopeak window should be used.
This is also the case when counting more than one radionuclide simultaneously. In this situation, the fractional detection in the window (W) is referred to as the photofraction, which is the fraction of the total number of pulses occurring in the spectrum that lie in the total energy peak. The photofraction increases with increasing crystal size and decreasing gamma-ray energy (figure 1.4(b)).
Typical counting efficiencies for commonly used radionuclides using a 3-inch diameter well-shaped crystal and an open energy window are shown in table 1.1.
Table 1.1. Counting efficiencies for some commonly used gamma-emitting radionuclides using a 76 mm long, 76 mm diameter well-type sodium iodide (Tl) detector with an open energy window.
| Radionuclide | Principal gamma energy (keV) | Efficiency (%) |
|---|---|---|
| 125I | 29 | 82 |
| 99mTc | 140 | 89 |
| 123I | 159 | 89 |
| 131I | 364 | 43 |
| 58Co | 810 | 65 |
| 59Fe | 1292 | 28 |
Gamma counters are normally used to assay sample counts relative to counts for a standard. By relating the standard counts to a traceable absolute measure of activity, the counting efficiency can be determined and therefore the sample activity can be calculated.
1.4. Liquid scintillation counting
Liquid scintillation counting (LSC) uses a liquid scintillator to convert energy from nuclear emissions into light photons which are detected by PMTs. The scintillator is known as a liquid scintillation cocktail and comprises a solvent and several solutes (often referred to as scintillants, fluors, or lumiphors); it is usually dispensed into a glass or plastic vial before the addition of a radioactive component. The vial is placed into a light-tight chamber known as a light guide, which is connected to (typically) two PMTs, and a spectral output is recorded. Liquid scintillation counting is very versatile, and the cocktail choice allows the efficiency to be optimised for both the chemistry of the radioactive component and the emission type. Typically, liquid scintillation cocktails are optimised to provide high counting efficiencies for alpha and beta emissions from liquid radioactive samples (often with weak acidic or basic chemistries); however, solid samples and other emission types are also commonly counted using this method. A detailed description of the physical principles and practical considerations of liquid scintillation counting is provided in chapter 6.
1.5. Solid-state detectors
Solid-state detectors are increasingly being used in nuclear medicine and health physics applications. Solid-state detectors, also known as semiconductor detectors, are made of solid materials with a crystalline structure. This crystalline structure has bandgaps of a few electron volts (eV). The majority of electrons in semiconductor materials are bound to specific sites in the lattice of the crystal within a low-energy state described as the valence band. When radiation is absorbed by a solid-state detector, ionisation occurs, which moves bound electrons out of the valence band and into a conduction band. In this high-energy state, the conduction electrons can flow freely, similarly to electrons in a metal. The vacancies left behind by excited electrons are known as holes, and they behave as positive charge carriers. Under the influence of an electric field, free electrons and holes drift in opposite directions towards opposite electrodes in the detector's surface, as shown in figure 1.5, generating an electric pulse.
Figure 1.5. Planar configuration of a semiconductor detector. A voltage is applied between cathode and anode. As radiation interacts with the detector material, electron–hole pairs are created, which drift as a result of the voltage, creating an electric pulse. Reproduced under the Creative Commons license (Zhang et al 2013).
Download figure:
Standard image High-resolution imageWhen radiation interacts with a semiconductor material, the number of electron–hole pairs formed is proportional to the radiation energy absorbed, which is, in turn, proportional to the amplitude of the electric pulse generated.
Certain semiconductor materials, such as germanium or silicon-based materials, need to operate in low temperatures (i.e. −196 °C), to suppress the formation of electron–hole pairs due to thermal vibrations. These operating conditions are not practical for most clinical applications. Instead, semiconductor materials with wide bandgaps are used clinically, as they can operate at room temperature without the need for cooling devices (Cherry et al 2012).
One such material is cadmium zinc telluride (CZT). This is a compound semiconductor with a high density and a high effective atomic number, resulting in high detection efficiency (Takahashi and Watanabe 2001). The direct conversion of incident radiation into an electrical signal reduces statistical noise, producing superior energy resolution to that of scintillation detectors. However, due to the low mobility of charge carriers, the timing resolution of CZT is inferior to those of scintillation detectors, and in addition, the semiconductor material cost is relatively high (Zhang et al 2013).
CZT is frequently used in intraoperative probes, as its high efficiency facilitates the detection of low levels of radioactivity, while its excellent energy resolution enables scatter rejection.
References
- Anger H O 1951 Scintillation counters for radioactive sample measurement Rev. Sci. Instrum. 22 912–14
- Birks J B 1964 The Theory and Practice of Scintillation Counting (Oxford: Elsevier)
- Blau M and Dreyfus B 1945 The multiplier photo‐tube in radioactive measurements Rev. Sci. Instrum. 16 245–48
- Cherry S R, Sorensen J A and Phelps M E 2012 Physics in Nuclear Medicine 4th edn (Philadelphia, PA: Elsevier Saunders)
- Hine G J (ed) 1967 Instrumentation in Nuclear Medicine (Cambridge, MA: Academic Press)
- Hofstadter R 1949 The detection of gamma-rays with thallium-activated sodium iodide crystals Phys. Rev. 75 796–810
- Kallmann H 1950 Scintillation counting with solutions Phys. Rev. 78 621–22
- NIST 2020 X-ray transition energies database (https://physics.nist.gov/cgi-bin/XrayTrans/search.pl?element=Pb&trans=All&lower=&upper=&units=eV)
- Raben M S and Bloembergen N 1951 Determination of radioactivity by solution in a liquid scintillator Science 114 363–64
- Takahashi T and Watanabe S 2001 Recent progress in CdTe and CdZnTe detectors IEEE Trans. Nucl. Sci. 48 950–59
- Zanzonico P and Heller S 2000 The intraoperative gamma probe: basic principles and choices available Semin. Nucl. Med. 30 33–48
- Zhang Q et al 2013 Progress in the development of CdZnTe unipolar detectors for different anode geometries and data corrections Sensors 13 2447–474





