Abstract
Nowadays, the deadly viruses (including the latest coronavirus) and pathogens transmission became the major concern worldwide. Efforts have been made to combat with these fatal germs transmitted by the airborne, human-to-human contacts and contaminated surfaces. Thus, the antibacterial and antiviral materials have been widely researched. Meanwhile, the development of diverse nanomaterials with the antiviral traits provided several benefits to counter the threats from the surface and airborne viruses especially during the Covid-19 pandemic. Based on these facts, this paper overviewed the advantages of various nanomaterials that can disinfect and deactivate different lethal viruses transmitted through the air and surfaces. The past development, recent progress, future trends, environmental impacts, biocidal effects and prospects of these nanomaterials for the antiviral coating applications have been emphasized.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The primary means to combat the growth of the bacteria and viruses on various surfaces such as the glass, wood or iron/steel are to avoid their early adhesion onto these surfaces [1–3]. In this view, different types of coatings have been developed including the chlorhexidine included hydroxyapatite, chlorhexidine blended polylactide, polymers and calcium phosphates plus chlorhexidine besides the nanomaterials such as copper (Cu) and silver (Ag) [1, 4]. Diverse nanomaterials due to their unique and novel properties became useful for the biomedical, biosensensing, optoelectronics and catalyses applications [3]. These distinct characteristics of the nanomaterials emerge from their reduced size or dimension typically below 100 nm [5]. Depending on the structures and morphologies, these nanosystems are classified into zero dimensional (0D) such as the nanoparticles (NPs) and quantum dots (QDs); one-dimensional (1D) for example the nanofibers (NFs), nanotubes (NTs), nanorods (NRs) and nanowires (NWs); two dimensional (2D) including the quantum wells (QWs) and films such as the graphene and graphene oxide [6, 7]; and three dimensional (3D) so called the bulk or macroscopic materials that are comprised of the equiaxed nanocrystallites with 3 random dimensions larger than nanometer [8]. In fact, some systems also exist in the boundaries of these classes of materials. In addition, the nanomaterials can be obtained naturally and prepared using various chemical, physical, biological and mechanical techniques [6, 9–11].
According to the International Organization for Standardization (ISO), each dimension of the nanoparticles (NPs) must be in the nanometer range. Conversely, for the NRs and nanoplates the length along the largest and smallest direction is appreciably different [8]. The NPs can be prepared with different sizes, shapes and dimensions such as the sphere, cylinder, cube, triangle, ring or disk [12–14]. Generally, these NPs are classified depending on whether they are composed of carbon, organic and inorganic compounds (figure 1). The organic NPs are normally biodegradable without any toxicity including the dendrimer, ferritins and hollow sphere (for instance the micelle and liposome). The inorganic NPs are mostly based on different metals such as the silver (Ag), gold (Au), iron (Fe), aluminium (Al), cadmium (Cd), cobalt (Co), zinc (Zn) and Cu as well as the metal-oxides including the TiO2, FeO2, Fe3O4, SiO2, CeO2, and ZnO2. All these organic and inorganic nanomaterials reveal emerging properties those are completely absent in their bulk counterparts [15, 16]. The carbon-based NPs can further be categorized into fullerene, graphene and CNTs [17, 18].
Figure 1. Classification of the nanoparticles based on their nature.
Download figure:
Standard image High-resolution imageThe NPs being the linkages among the bulk structures as well as the atomic and molecular structures, their emergent novel characteristics mainly depend on the individual components. Consequently, the overall attributes of the NPs are appreciably different than the one exist at larger dimensions. Regardless of their nature, the most important properties that lead to the extensive uses of these NPs are related to their enlarged surface area, emergent optical traits due to the quantum size effects, improved absorbance, uniformities, and surface functionalizations. Especially, the effect of quantum confinement in the NPs leads to the emergence of the spontaneous semiconducting, conducting or insulating properties for the adjacent particles [19], improved stabilities, chemical and physical behaviours [17].
In recent times, the interests of using the NPs for varieties of biomedical applications have been ever-growing. Usually, the surfaces of the antimicrobial materials contain some chemical reagents responsible for inhibiting the microorganisms growth [20]. Materials with such surfaces have widely been studied for various clinical, industrial and domestic applications. The main uses of these materials are in the medical sectors as the anti-microbial coating and sterilizations of various equipment to prevent the infection spread in the hospitals that are responsible for numerous annual deaths in the US [21]. Beside the medical equipment, the clothes are the ideal environments for the growth of many germs. Upon contacting the patients, these infected clothes enable an easy spread of these diseases [22]. To overcome such spread of the diseases, the antimicrobial surfaces have been functionalized through varieties of strategies. For instance, the applied coating to the surface may be the chemicals that have toxicity effects to different microorganisms. Furthermore, via the attachments of various polymers or polypeptides on the frequently used surfaces they can be functionalized to prevent the microbial infections [21].
An innovation in the antimicrobial surfaces began with the discovery of diverse nanomaterials such as Cu and its alloys (brass, bronze, cupronickel, copper-nickel-zinc, and others), Ag, Au, Ti and Zn. These materials due to their unique anti-microbial traits can destroy varieties of micro-organisms [23]. A recent review indicated the strong anti-microbial effectiveness of Cu against the E. coli (O157:H7), methicillin-resistant S. aureus (MRSA), Staphylococcus, Clostridium difficile, influenza A virus, adenovirus and fungi [24]. Apart from the medical and health-care sectors, the anti-microbial surfaces have also been used by other industries because of their self-cleaning traits. In fact, both physical and chemical characteristics of different surfaces can be customized to develop the anti-microorganism environments useful towards specific applications. Earlier, many photocatalytic materials have been utilized to kill several microorganisms. Lately, these photocatalytic materials became useful for the self-cleaning surfaces, air cleaning, water purification and antitumor activity [25].
Considering the immense benefits of the nanomaterials-integrated antimicrobial surfaces, this article comprehensively overviewed the potential of several nanomaterials that can disinfect as well as deactivate viruses and pathogens transmitted through the air and contaminant surfaces. The antimicrobial efficacy of these nanomaterials is presented in terms of their types, surface morphologies, microstructures, fabrication procedures and biocidal performances. In addition, an all-inclusive list of various nanomaterials is provided that are known to deactivate the deadly viruses on the common surfaces and protect humans against the infectious diseases transmission. In short, the past developments, recent progress and future prospects of these nanomaterials together with their environmental impact and biocidal effects and uses as the anti-viral agents are underscored.
2. Nanomaterials-based antimicrobial coatings
Any virus is comprised of a group of highly heterogeneous and simple organism with the size range of 100–300 nm (considerably smaller compared to the bacteria) [26]. The viruses do not have any independent metabolic activities and entire depend on the living hosts for the reproduction. Unlike other living microorganisms, they enclose either DNA or RNA as the genetic components. The nuclear components in the viruses are surrounded by some layer of proteins for the protection from harmful agents present in the surroundings. In addition, such coating provides the docking sites required for attaching the viruses to their hosts. Some viruses possess an external cover composed of the lipid, polysaccharide and protein molecules that enable the viruses to combine with the host cells, thus facilitating the entrance into the hosts. However, the viruses devoid of such external envelopes can also infect the host cell via other means. The viral diseases are commenced through the attachments of the special sites of the virus protein-coated surfaces on the specific receptor sites of the host cell surfaces [27, 28].
The viral attacks can be inactivated by controlling the surfaces of the viruses particularly the docking sites, making the receptor site of the host cells totally unrecognizable. The coatings of diverse nanomaterials can effectively be used to inhibit the virus attack, thereby offering a viable solution to destroy the surface structures of the viruses. Currently, the antimicrobial, antiviral, and antifungal nanocoatings on the surfaces with various material compositions are available for the healthcare, household, inside and outside uses that protects the decay and mildew together with water and air cleansing. In addition, the coatings of the nanomaterials are used to reduce the surface contaminants, improve the self-cleanliness, enhance the water repellent tendency, improve the smell inhibition, and cost saving maintenances. The antimicrobial, antiviral and antifungal nanocoatings may be obtained via the spray, sinking and attachments on various surfaces of the glasses, metals, alloys, marbles, stones, ceramic tiles, textiles and plastics [29].
Generally, Covid-19 and other influenza viruses are transmitted among people via the airborne respiratory droplets escaped during the coughing or sneezing. Such infections are spread when a healthy person come in contact with these droplets that stick on the frequently used objects or surfaces [30]. Thus, an antiviral self-disinfecting surface can play a decisive role to destroy such viruses from further spreading. The glass surfaces coated with the hydrophobic (N,N-dodecyl, methyl-PEI) show strong antiviral traits against the lethal waterborne influenza A viruses including the wild-type human and avian strains in addition to their neuraminidase mutants resistant to the anti-influenza medicines [3]. In this regard, the nanotechnology and nanoscience with the immense biomedical potential can be effective for the self-disinfection of various surfaces. Being comparable to the nature, the combined nanoscience and biology can support to resist the pathogens, thereby offering an alternate strategy to combat against the contagious diseases [31].
Of late, the biological characteristics of various metallic NPs have been explored via the antimicrobial susceptibility tests. These NPs have been produced using different techniques. Some reports suggested that the metal NPs (silver, copper, copper oxides and gold) have a broad range of the anti-microbial activities against diverse fungi and bacteria. The bactericidal action of these NPs against the E. coli [32] and S. aureus [33–35] has been reported. The uses of the proposed NPs were shown to inhibit these microorganisms growth. The metallic NPs generally disclose the antibacterial and antifungal activity despite the safety concern of the environment and human health related to the discharge and utilization of these NPs, which is an emerging area that needs further exploration. It was found that the surfaces coated with the nanomaterials of Cu, Ag and Au can prevent the infection spread in the healthcare environments [36]. The microorganisms are known to survive on the inanimate surfaces for an extended time period [36]. Thus, the disinfection practices for the hands and surfaces became a primary measure against the microorganisms-mediated infections spread. Approximately 80% of the infectious diseases are transmitted by touching the surfaces wherein the pathogens are found to survive on the inanimate surfaces for a prolonged time duration (days or months) in the healthcare facilities [37]. Thus, the microbial burden of the frequently touched surfaces play a substantial role in the infection causalities [38]. In this perception, the nanomaterials can significantly address several clinical and public healthcare challenges emerged from Covid-19 pandemic. Based on these factors, we analyzed the usefulness of the nanotechnology and nanomaterials fighting against the Coronavirus and ongoing mitigation strategies. The nanostructured materials-based products are currently being developed and deployed for the inhibition, diagnosis, and treatment of Covid-19.
Following the nanotechnology route, Michielsen had modified the surfaces of the polymers/fibres using a thin dye coating [39]. This coating acted as the photo-catalyst agent when exposed to the incident light, sparking a chemical reaction in the air that could kill most of the viruses and bacteria. A specific reaction occurred on the surface in the presence of light, making the air poisonous to the microbes without causing any harm to human. In addition, the coating did not wear out and regenerated continually to kill the viruses (figure 2). The Canadian researchers have developed a clear surface coating called the NanoClean that could kill the viruses (including the Coronavirus) upon contact and lasted for several weeks [28]. This self-sterilizing nanocoating is among the latest Canadian technologies. Recent studies revealed that the SARS-CoV-2 that causes the COVID-19 can live on the surfaces for 72 h and thus spread rapidly. The NanoClean was shown to kill up to 99.9% of the viruses and bacteria when applied on the surfaces. Moreover, its germ-fighting potency can be triggered by the light energy thereby providing a longer protection against the surface-to-contact transmission than the conventional sterilizers. On top, it can be used on the high-touched surfaces such as the plastic chairs, doorknobs and handrails to reduce the spread of Covid-19 [40].
Figure 2. Illustration of the nanocoating technology working [40].
Download figure:
Standard image High-resolution image3. Types of nanomaterials for antimicrobial coatings
Over the years, various NPs have been used for different antimicrobial applications due to their distinctive characteristics [3]. Highly reactive nature of the nanomaterials offers their usage in the antimicrobial coatings [41]. Repeated studies indicated that various metallic and metal-oxides NPs such as the ZnO, CuO, Ag, CuI, Au–SiO as well as some quaternary ammonium cations are advantageous for the virucidal applications [42, 43]. Amongst all the metal-based nanomaterials with the antimicrobial traits, the Ag and Cu NPs shows the best antiviral activities [43, 44]. The anti-HIV activity of the Ag NPs [45, 46] was argued to be most likely due to their direct binding with the gp120 [47]. Meanwhile, the Ag NPs can hinder the growth of the viruses such as herpes simplex type 1 [48] and type 2 [49], vaccinia [50], respiratory syncytial [51], influenza A [52], tacaribe [53] and hepatitis B [47, 54]. The upcoming sections describe the applications of various nanomaterials for the antiviral surface coatings.
3.1. Copper (Cu)
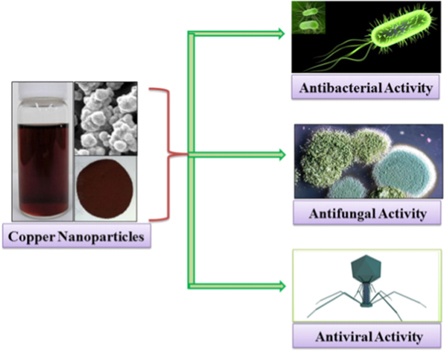
The abundance of Cu and its various properties similar to the expensive noble metals (Ag and Au) make it a better choice for the antimicrobial applications. Recently, the Cu NPs generated renewed interests because of their catalytic, optical and antimicrobial applications (figure 3). The antimicrobial activities of the Cu NPs against several bacteria and fungi have been investigated [4, 23]. Some studies indicated the antimicrobial activity of the Cu NPs against the E. coli and Staphylococcus species [55] with comparable antifungal attributes [56]. However, the rapid oxidation of the Cu NPs under the exposure of open air remains the major limitation. During the Cu NPs synthesis, the element Cu is oxidized to the CuO and Cu2O, resulting in Cu2+ that makes the Cu NPs preparation difficult at an ambient environment. To overcome this problem, other strategies have been adopted to synthesize the Cu NPs wherein different polymers and surfactants are used as the stabilizing agents. In addition, the extracts from different plants have been utilized in the green synthesis techniques for stabilizing the as-prepared NPs [57]. The preparation of numerous types of NPs using the polymer dispersions have been reported [58]. Diverse techniques for the syntheses of the Cu NPs have been introduced including the thermal reduction [59], capping agent, sonchemical reduction, metal vapour [60], micro-emulsion [61], laser irradiation [62] and induced radiation.
Figure 3. Schematic representation for the antimicrobial activity of Cu NPs [63].
Download figure:
Standard image High-resolution imageThe antimicrobial mechanisms of the Cu NPs have intensively been investigated [64]. The antimicrobial traits of the Cu NPs can be attributed to various mechanisms including the (i) elevated Cu levels inside a cell cause the oxidative stress and generation of the hydrogen peroxide, wherein Cu participates in the so-called Fenton-type chemical reaction responsible for the oxidative damage of the cells; (ii) excess Cu concentration causes the decay in the membrane integrity of the microbes, leading to the leaking of certain necessary cell nutrients such as the K and glutamine which in turn cause the desiccation and successive cells death; (iii) for several performances the proteins Cu is required, however its excess amounts lead to the bindings of some proteins that are unnecessary for their function where such unwanted binding can lead to the improper working of these proteins and/or collapse into non-functional parts; (iv) continuous reduction of the bacterial attacks occurs, attaining 99.9% decrease within 2 h of the application where more than 99.9% of the Gram-negative and Gram-positive bacteria are killed within 2 h of the exposure. The presence of the Cu NPs delivers nonstop bactericidal activities on the residual living bacterial strains thereby destroying above 99.9% of them in 2 h, killing over 99.9% of remaining bacterial strains in 2 h and persist to destroy 99% of these strains still after the continual attacks; (v) it helps to inhibit the build-up and bacterial growth in 2 h of the exposures between router cleaning and sanitization stage.
The antiviral efficacies of the Cu alloy surfaces have widely been investigated [65]. It was demonstrated that after the incubation for 1 h on Cu, the effective influenza A viruses can be diminished by 75% and after 6 h they it can be reduced by 99.99% [66]. In addition, about 75% of the Adenoviruses can be destroyed on the Cu in 1 h and 99.999% can be killed within 6 h [67]. The Cu NPs in the size range of 9.25 ± 1.79 nm were prepared using the wet-chemical process. The antifungal effectiveness of the Ag and Cu NPs were compared by measuring the inhibition zones diameter (IZD) using the disk-diffusion tests. The Ag NPs revealed higher bactericidal effectiveness against the E. coli and S. aureus bacterial strains than the Cu NPs. Conversely, the Cu NPs showed better antibacterial potency against the B. subtilis than the Ag NPs. The antifungal activity of the Ag and Cu NPs against the Trametes versicolor fungus was examined [68]. Both NPs revealed considerable decrease in the mass loss, whereas the Cu NPs displayed better antifungal effects than Ag NPs. In addition, the hardness values of two prepared panels were dropped radically after the exposure to the fungi wherein the Cu NPs-treated panels revealed a significant improvement in the hardness. Several studies [69–72] have been conducted to evaluate the antimicrobial potency of the commercial Cu NPs against the E. coli and B. subtilis bacteria. The results showed a decrease in the survival fraction of the bacteria with the increase in Cu NPs contents. In short, the replication of the bacteria was totally inhibited when the contents of Ag and Cu NPs were above 70 and 60 lg/ml, respectively.
The antibacterial Cu NPs coating can be used in the hospital, clinic or home to prevent the spread of the infections especially for the old people and infants at higher risks. The antimicrobial effectiveness of the nanoCu as additive in the architecture paints and impregnates was assessed [73]. The antimicrobial properties of the silica nanosphere integrated with immobile Cu NPs displayed the complete growth inhibition of the algae on the paints containing 0.5 ppm of the Cu NPs was while control samples were intensively covered by the algae. In addition, the tested surfaces covered with the paint containing Cu NPs at the concentration of 0.1 ppm showed a decrease in the micro-organisms growth by approximately 10%. The coatings containing the NPs of the safe metal ions and polymers displayed the excellent anti-viral and anti-microbial features that remained effective for weeks or even months [4]. The anti-viral coatings based on the Cu and other NPs-embedded polymers were used to paint/spray the surfaces. These NPs could control the release of the metal ions onto the coated surface, displaying their strong anti-viral effect via the eradication of the virus particles that adhered to the surface. An extremely slow release of the ions in the coating was greatly effective for the prolonged duration (over the weeks and even months), inhibiting the virus particles growth over tenfold.
3.2. Silver (Ag)
The antimicrobial traits of Ag are well-known to the human since ages. The Phonecians stored water and other liquids in the silver coated bottles to avoid the contamination by the microbes. Silver dollars used to be put inside the milk bottles to keep the milk fresh. The water tanks of ships and airplanes were silvered to keep the water drinkable for months. Later, it was realized that amongst all antimicrobial metallic systems, the element Ag has highest bactericidal efficiency and lowest toxicity to the animal cells [74]. Earlier, it was believed that the atomic Ag binds to the enzymes' thiol group (-SH) and then deactivates them. The element Ag makes the S-Ag bond with the compound made of thiols responsible for the trans-membrane energy production and ions transportation in the cells [75]. In addition, the Ag atoms can participate in the catalytic oxidation reactions and generate the disulfide bonds (R-S-S-R) via the catalytic reactions among the O2 in the cells and H in the thiols groups. In this process, H2O is produced and 2 thiol groups make the covalent bonds via a disulfide linkage [76]. The Ag catalysis-mediated disulfide bond generation can alter the shapes of the enzymes in the cells and thereby affects their functions.
The Ag nanosystems are considered as the novel kinds of the bactericidal materials with excellent potency for the surface coating in the medical equipment, foods packaging and industrial piping. Therefore, the Ag NPs have intensively been investigated as the potential antimicrobial materials. The simple syntheses and strong antibacterial activities of the Ag NPs make them attractive for the future drug formulation. Martinez-Castanon et al [77] prepared Ag NPs of different sizes (7, 29, and 89 nm) and determined the NPs size-dependent antibacterial effectiveness. In the syntheses technique, Ag nitrate was used as the Ag ion source and gallic acid was utilized for the reduction and stabilization of the NPs. The NPs of various diameters were obtained by changing the solutions pH and UV irradiation energy. The TEM images were recorded to determine the NPs morphologies and average diameters. The NPs size-dependent bactericidal activities were assessed via the MIC assays against the E. coli (Gram negative) and S. aureus (Gram positive) bacterial strains. The smaller Ag NPs revealed stronger bacterial growth inhibitory traits than the larger NPs wherein the S. aureus bacteria were more resistant to the Ag NPs than the E. coli. These findings agreed well with the observation made by Kawahara et al [78]. The observed enhanced antimicrobial activity of the smaller NPs was attributed to their relatively easier penetration through the bacterial cell membrane and cell wall than the larger NPs [77]. Briefly, the tinier Ag NPs has higher number of atoms at the surface to get in touch with the solution compared to bigger ones. The results indicated that more Ag atoms from the smaller NPs were able to participate in the cell destruction processes compared to the larger Ag NPs. Assuming that only the atoms from the outer silver layer of the NPs were ionized, the larger NPs could produce less silver ions than a the smaller one. In fact, the number of Ag ions was responsible for imparting the antibacterial properties to the NPs, thereby making the smaller Ag NPs more bactericidal than the larger Ag NPs. In addition to the sizes, the shapes of the NPs play the role to decide their bactericidal action.
Pal et al [79] prepared the Ag NPs of different shapes (spherical, rod-like and triangular) to determine their antimicrobial activity against the E. coli bacteria via the agar diffusion test, where the agar plates enclosed 1, 12.5, 50 and 100 μg of the above 3 kinds of Ag nanostructures as well as silver nitrate (AgNO3). The numbers of the colonies produced under each condition were recorded and plotted against the Ag concentration for all the four types of treatments. The triangular Ag NPs showed the strongest antibacterial activity followed by the spherical Ag NPs, rod-shaped Ag NPs and AgNO3. The observed trend of the bactericidal potency of the Ag NPs and AgNO3 was due to the presence of different facets of the NPs. The triangular Ag NPs had higher number of active faces (with dense electrons) compared to the spherical NPs, thereby showed better bactericidal efficacy than the spherical NPs. Conversely, the spherical Ag NPs facets were somewhat non-spherical, but had higher number of the active faces compared to the rod-like NPs [79]. This clearly indicated the significant role of the Ag NPs with different shapes on the antibacterial activity. In short, the NPs with higher number of the active facets showed stronger bactericidal action.
The Ag powders with the ultra-fine and uniform particles size distributions are advantageous for the surface coating. Being a safe, non-toxic to animal cells and effective bactericidal (highly toxic to the bacteria such as the E. coli and S. aureas) the metallic Ag became greatly demanding in all the technological sectors [80]. In recent years, various Ag-based compounds have broadly been used for the bacterial growth inhibition in the burn care applications [81]. In addition, the Ag-doped polymer fabrics, catheters and polyurethane have been deployed for their notable antibacterial functionality [82]. Additionally, the colloidal silver [83], nanoAg coated fabric [1], nanoAg metal oxide granules [84] and nanoAg coated ceramic materials [85] have been used for the antibacterial applications. Owing to their large surface area to the volume ratios, capacity of loading at small quantities and cost-effectiveness, the nanoAg powders and suspensions have extensively been used for widespread applications. Dedicated efforts have been made to produce monodispersed nanosized Ag powders using different approaches. Pluym et al [86] prepared nanoAg powders via the spray pyrolysis technique at the production rate of 1–2 g h−1. The produced silver NPs revealed agglomeration tendency, irregular shape and hollow types due to the solvent evaporation.
It is established that the antibacterial potency of various nanomaterials is mainly because of their wide surface areas and diverse morphologies (sizes and shapes) dependence of the physic-chemical characteristics [87–90]. Amongst different bactericidal nanosystems the nanoAg is considered to be the best because of its superior bactericidal potential compared to the bulk Ag [91, 92]. Additionally, the nanoAg has the wide range of bactericidal activates against various bacterial strains occur in daily life, aggressive environment, and industrial processing such as the antibiotics resistant bacteria [93]. Thus, it is essential to introduce the nanosilver as the antibacterial coating on various materials' surfaces for the widespread applications. Yet, the fabrication of the biocompatible coatings on the surfaces with high bactericidal effectiveness and environmental friendliness from the nanoAg remains challenging [94, 95]. The significant reduction in the antibacterial activities of the nanosilver can be ascribed to the aggregation of Ag NPs, uncontrollable release of the Ag ions and improved adhesion of the bacterial strains [96, 97].
Polymer-based compounds due to their excellent structural properties customization and flexibility reveal strong inhibition potency to the agglomeration of the nanoAg and formation of the homogeneous coating on the surfaces of various substrates [98]. In addition, such systems are able to regulate the discharge of the Ag ions for the constant bactericidal actions with low cyto-toxicity [99]. Again, these materials can be tailored to inhibit the bacterial attachments, thereby enhancing the antibacterial effectiveness [100, 101]. Therefore, it is beneficial to integrate the nanoAg with the polymers to create the multi-functional coatings of the nanocomposites for the potential bactericidal applications. Accordingly, the polymer and nanoAg nanocomposites having varied morphologies particularly the fibres were extensively investigated [102–104]. Recently, the antimicrobial properties and different functions of the Ag/polymer nanocomposites have been reviewed [105].
3.3. Gold (Au)
The Au NPs with modified surfaces became increasingly popular materials because of the feasibility of certain targeted drug delivery with enhanced biocompatibilities. Due to their unique physical and optical characteristics the Au NPs with the size range of 1 to 100 nm became promising for diverse biomedical applications. Generally, the organic materials or capping agents have been used to coat the prepared Au NPs to get their higher stability. The Au NPs reveal easy functionalization with other components such as the antibody, protein, ligand, DNA, polymer and polyelectrolyte [106–108] which are very useful for diverse biomedical applications.
Numerous techniques have been developed to synthesize the Au NPs with the controlled morphologies and surface properties [109, 110]. Following the Turkevich technique, the compound HAuCl4 was treated with the citric acid inside boiled water wherein the citrate acted as both reduction and stabilization agents [111]. It produced the Au NPs with the diameters range of 10 to 20 nm, where the diameters were tailored by adjusting the ratios of the gold to citrates contents [112]. The alkanethiol-stabilized Au NPs suspension in the organic solvents was synthesized using the tetraoctylammonium bromide (TOAB) and NaBH4 as the capping and reducing agents, respectively [113]. Controlling the Au contents to thiol proportion, temperatures, and reduction rates, the NPs with the diameter range of 1.5 to 5 nm were produced [113]. In addition, various reduction agents including the sucrose, ethylenediaminetetraacetic acid, fruit extract and amines were used to produce Au NPs with uniform size and narrow distributions [114, 115].
Due to their large specific surface area and easy functionalization feasibility with diverse chemical functional groups, the Au NPs and C QDs became the main candidates for the interaction with the virus to prevent their infection in the human cells. Of late, some studies (University of Lille of France and Ruhr University of Germany) on the functionalizations of the C QDs with boronic acid ligands revealed their interference with the functions of the S proteins of the coronavirus and significant inhibition of its penetration in the host cells. The evaluation showed that the inclusion of such nanosystems into the cells culture before and during the infections with the coronavirus can greatly lower the infection rates of the cells. Unexpectedly, after 1 life-cycle (approximately 5.5 h) of the coronavirus a significant growth inhibition was found at the virus replication stage. The synthesized C QDs with mean size of 10 nm and outstanding water solubility were shown to be ideal to kill the coronavirus. The observed vrucidal efficany was ascribed to their easy entry through the cell membranes via the endocytosis and interaction with the virus proteins, thus inhibiting the virus genomes replications [116, 117].
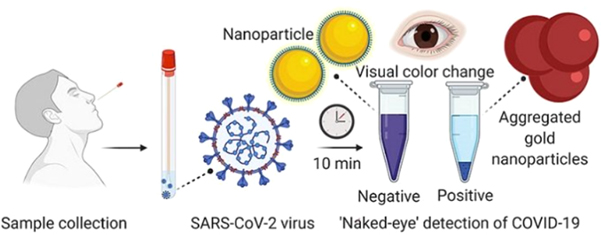
Similar antiviral effects of other nanomaterials have also been demonstrated [108]. For instance, a novel class of Au nanorods-based peptide inhibitors has been developed that could selectively target the S protein of the coronavirus to disrupt its activity. Lately, scientists from the University of Maryland (School of Medicine) have developed an experimental diagnostic test for the Covid-19 that can visually detect the presence of the virus in 10 min. A simple assay containing the plasmonic Au NPs was used to detect the color change when the virus is present. This test does not require any advanced laboratory techniques that are commonly used for the DNA amplification analysis. Once a nasal swab or saliva sample was obtained from a Covid-19 patient, the RNA was extracted from the sample via a simple process within about 10 min and the test used a highly specific molecule attached to the Au NPs to detect a particular protein. This protein was a part of the genetic sequence that is unique to the novel Coronavirus. As illustrated in figure 4, the Au NPs can respond by turning the liquid reagent color from purple to blue when the biosensor binds to the virus gene sequence.
Figure 4. The nasal swab with a test sample subjected to the examination. A liquid was added to the Au NPs bound to a molecule that adhered to the novel coronavirus. The Au NPs turns the solution a deep blue color (bottom of the tube) when the viruses are present followed by a precipitation. The solution retained its actual purple color in the absence of the coronavirus.
Download figure:
Standard image High-resolution image3.4. Silica (SiO2)
The nanotechnology may be used to fight against the microbial infections via the synthesis of various antimicrobial agents [118–120]. In this regard, silica is another inorganic and bio-friendly material used for the antiviral coating [121]. The extreme chemical stability and easy preparation of silica makes it widely popular with several notable advantages. The surface enrichment of the silica via the silanol groups enables easy reaction with the coupling agents, thereby providing strong attachment of the surface ligands on the metallic NPs (MNPs) [122]. In addition, the silica coating can enhance the stabilization of the MNPs in the liquid dispersions by preventing the dipolar attractions and increasing the number of surface charges, hence improving the electrostatic repulsions amid the particles in the non-aquaeous dispersions. Numerous methods have been developed to create the silica coating. The Stöber method is the most popular one which is based on the hydrolysis reaction of the tetraethyl ortosilicate (TEOS) in the alcohol media under the catalysis by ammonia [123]. Following the Stöber method, Deng et al [124] prepared silica coated iron oxide core–shell NPs, where the condensation of the TEOS in the sol-gel form on the pre-formed magnetite NPs was used. The morphologies and coating thickness of these core–shell NPs was systematically tailored by adjusting the types and amounts of the alcohol, ammonia, and TEOS. Santra et al [125] utilized the water-in-oil microemulsion method to coat the iron oxide NPs wherein different nonionic surfactants was used to obtain the NPs in the size range of 1–2 nm with uniform distribution (standard deviation below ±10%). Yi et al [126] used a reverse microemulsion method to produce some homogenous silica-coated SiO2/Fe2O3 NPs with various shell thicknesses. Finally, the mesoporous silica-coated SiO2/Fe2O3 MNPs and hollow SiO2 nanoballs were obtained [126]. The influence of coating thickness on the magnetic properties of the iron oxide NPs was determined. The silica encapsulated Au-coated MNPs were attached with different functional components via the embedment or binding onto the silica shell to realize multifunctional nanosystems [127]. These NPs were claimed to be useful for various practical applications including the infectious diseases treatment, cancer therapeutics, vaccine deliveries and consumer food products [128, 129].
3.5. Titanium dioxide or titania (TiO2)
Usually, the decontamination of the surfaces is carried out repeatedly using the disinfectant solution to reduce the possibility of the recontamination. Recently, photocatalytic antimicrobial coating technology has been introduced as an alternative because of its long lasting effect compared to the one time use of the disinfectant solution [14]. The titanium dioxide or titania (TiO2) has been used as the antimicrobial coating due to its strong photocatalytic properties [69, 130]. The mechanisms of the TiO2 photocatalysis involve the generation of the electron- hole (e−-h+) pairs that diffuse and eventually get trapped on or near the TiO2 surface in the presence of light [131]. The generated electrons and holes due to their strong reduction and oxidation potential can react easily with the atmospheric water and oxygen, creating hydroxyl radicals (OH−) and superoxide anions (O2 −). These radicals are extremely reactive to the organic compounds and bacterial cells, producing complete oxidation and depletion [131]. These properties of titania is exploited to disinfect the surfaces, air, food and water as well as to produce anti-fogging and self-cleaning coatings on the glass and pharmaceutical additives [68]. The nanoscale TiO2 has been claimed to be the excellent coating material for the antiviral, antibacterial, anti-static, anti-odor, reduction of the volatile organic compounds (VOCs) and UV radiation protection [3]. Some medical development divisions reported the strong antibacterial efficiency (in the range of 30 to 95%) of the TiO2 photocatalyst coatings against the E. coli and S. aureus [132]. Conversely, the TiO2 photocatalyst is not that effective against some spore forming bacteria (such as the Bacillus atrophaeus) and fungal species (for example Aspergillus Niger and yeasts).
Min et al [133] conducted a prospective cohort study involving 621 patients in the medical intensive unit. The titania-based photocatalyst was coated on the high touch surface and walls. Some comparison of the multidrug resistant organism incident rates, hospital-acquired blood stream infection, pneumonia, urinary tract infection and Clostridium difficile diseases were performed between the pre-intervention and post-intervention (five months of data each set). The results revealed a significant decrease in the MRSA acquisition rate after the photocatalyst coating (hazard ratio of 0.37, 95% confidence interval (CI) of 0.14 and 0.99 with p = 0.04). However, the clinical identification of the vancomycin resistant Enterococcus spp. and multidrug-resistant Acinetobacter baumannii did not display any significant reduction. The hazard of the hospital-acquired pneumonia during the intervention period compared to the baseline period was 0.46 (95% CI of 0.23 and 0.94 with p = 0.03) [133].
Matthew et al [134] examined the effectiveness of the TiO2 coating to reduce the bioburden for the high risk surfaces in between two acute care wards. The TiO2 was sprayed onto six surfaces as coating agent and compared under the normal illumination against the same surfaces in an untreated ward with right and left bed rails, bed control, bedside locker, overbed table, and bed footboard. The general microbial burden and presence of an indicator pathogen (Staphylococcus aureus) were evaluated biweekly for the period 12 weeks. The titania treated surfaces demonstrated a significant lowering of the microbial burden than the control sites wherein the difference between the treated and untreated surfaces was increased during the study. The hygiene failures (>2.5 colony-forming units [CFU]/cm2) for the control surfaces were increased by 2.6% per day (odds ratio [OR] of 1.026, 95% CI of 1.009 and 1.043 with p = 0.003). However, the hygiene failures for the treated surfaces were declined by 2.5% per day (with OR of 0.95, 95% CI of 0.925 and 0.977 with p < 0.001) [135].
The coating materials with anti-microbial photosensitizers have advantages than the titania that is normally used as an antibacterial agent but ineffective against viruses. Following the approach of the Spontak team, both bacteria and viruses can be killed. The UV radiation is required to activate the titania that has detrimental effects on the healthy cells. Moreover, the use of the NPs has increasingly been scrutinized for the potential human health hazards during their exposures. The photoactivation of the titania by the UV radiation generates the reactive oxygen molecules on the TiO2 surface that has been shown to effectively inactivate the influenza A virus [136], HIV-1 [137] and murine norovirus [138]. The halogen and interhalogen TiO2 NPs, except for the chlorinated adduct can completely inactivate the bacteriophages MS-2, ψ-X174 and PRD-1, displaying their oxidizing potential that can be generated without the UV photoactivation [139]. Similarly, the metal oxide NPs, CeO2, Al2O3 and their halogen adducts exhibit excellent antiviral activities against the bacteriophages.
3.6. Zinc oxide (ZnO)
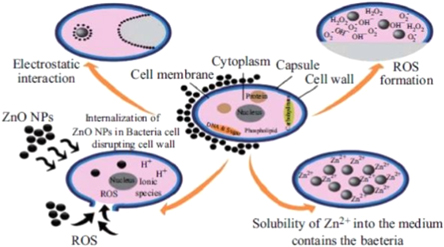
Generally, the semiconducting ZnO is accepted to be biocompatible without any toxicity to the human [140]. However, some of the recent reports revealed certain toxicity effects of the ZnO NPs due to its photocatalytic activity [141, 142]. Amongst all the metal oxide NPs developed so far, the ZnO NPs display the highest toxicity against the microorganisms [143]. The SEM and TEM image analyses of the ZnO NPs showed that these NPs first damage the bacterial cell wall followed by the penetration and subsequent accumulation in the cell membrane. These NPs can interfere with the metabolic activities of the microbes, thereby leading to their deaths. Various properties of the ZnO NPs are decided by their morphologies, concentrations, and exposure times to the bacterial cells. The ZnO NPs absorb the UV radiation and promotes the interactions with the molecules present in their immediate vicinity [144]. This photocatalytic process can continue long after their activation by the UV light absorption. The photocatalytic traits of the ZnO NPs can be attributed to the depletion of the surface electrons connected to the adsorbed negatively charged oxygen derivatives on the NPs surface. Upon activation with the UV light in the presence of oxygen, the ZnO NPs in the aqueous dispersions show a phototoxic action due to the formation of the reactive oxygen species (ROS). This in turn leads to the generation of the hydrogen peroxide (H2O2) that has a strong antimicrobial effect. Thereafter, the generated ROS can diffuse into the microbial cells and damage the interior as well as the cell walls, thus inhibiting their growth. The photocatalytic effect underpins the understanding of the antimicrobial action of the ZnO NPs in the drug formulations and other nanotechnology-based applications. The antimicrobial activity improvement of the ZnO NPs is due to the creation of more free radicals upon the activation by the UV light. Several researchers [142, 145] have discussed the possible reaction mechanism of this effect. Nagarajan and Rajagopalan [146] established a correlation between the photon-initiated process of the photocatalytic NPs and their antimicrobial effect. Figure 5 shows the typical relationship amid the effects of the important parameters of the ZnO NPs on the bactericidal responses and feasible mechanisms such as the ROS generation, Zn discharge, ZnO NPs entry into the bacteria and electrostatic interactions [142].
Figure 5. Various bactericidal mechanisms of the ZnO NPs [142].
Download figure:
Standard image High-resolution imageThe photocatalytic titania has extensively been used due to its nontoxicity, high activity, strong self-cleaning traits and low cost. The photocatalytic process of the TiO2 or ZnO involves the generation of the electron-hole pairs during the light exposure where the generated holes react with the nearby molecules to produce the oxidants. Then, the hydroxyl radicals (OH−) are produced due to the chemical reaction of these particles with water and oxygen, creating the superoxide molecule. Similar to the titania, the ZnO and other semiconducting materials show unusual electronic and chemical properties useful for inhibiting the growth of the microorganisms [147–149]. Eventually, these radicals attack the bacteria or viruses via the inhibition of the DNA clonal processing, destroying the coenzymes and enzymes through the self-regeneration in the respiratory system. Consequently, the radical stops the reproduction of the bacteria and molds, thereby inhibiting the bacteria growth or preventing the virus DNA multiplication [150, 151].
The morphology of the ZnO nanostructures is strongly sensitive to the synthesis techniques. So far, diverse nanomorphologies of the semiconductor ZnO have been developed. These nanomorphologies include the nanorods, nanoplates [152–154], nanospheres [155], nanoboxes [154], hexagonal, tripods [156], tetrapods [152], nanowires, nanotubes, nanorings [157], nanocages and nanoflowers [158]. Amongst all these nanostructures, the ZnO NPs are more active against the gram-positive bacteria compared to other NPs made of the elements from the same group in the periodic table. Ready to eat food is more susceptible to the infection by Salmonella, Staphylococcus aureus, and E. coli which pose a great challenge to the food safety and quality maintenance. Various antimicrobial compounds have been incorporated in the packed food to prevent them from the bacterial damage and decomposition. The antimicrobial packaging contains some nontoxic materials that inhibit or slow down the growth of the microbes present in the foods or packaging components [159].
The ZnO is one the inorganic metal oxides that fulfills all the requirements of the antimicrobial food packaging. Thus, the ZnO NPs can safely be used as the medicine, preservative in packaging and antimicrobial agent [160, 161]. The easy diffusion of these NPs into the food products helps to kill the microbes and prevent the human being from falling ill. According to the 1935/2004/EC and 450/2009/EC regulations of the European Union, active packaging is defined as the active materials contact with the food that can change the composition of the food or its surrounding atmosphere [162]. Therefore, it is commonly used as preservative and incorporated in the polymeric packaging material to prevent the microbes' related damage of the food materials [163]. The ZnO NPs have been used as the antibacterial substance against the Salmonella typhi and S. aureus in vitro.
The nanoscale ZnO has been demonstrated to exhibit diverse morphologies and significant antibacterial activity for varieties of the bacterial species [164, 165]. This wide band gap semiconductor material displays strong antimicrobial action when the particles dimension is decreased to the nanoscale. The nanoscale ZnO interacts strongly with the surfaces and/or with the cores of the bacteria by entering within the cell and reveals unique antibacterial actions [166]. The interaction amid the unique nanostructures and bacterial strains are typically toxic, and thus suitable for the antimicrobial applications especially for the surface coatings. Interestingly, the ZnO NPs are nontoxic to the human cells [167] but toxic to the microorganisms. This distinct characteristic of the ZnO NPs enables their use as the bactericidal agents because of the noxious to the micro-organisms and biocompatible nature to the human cells [146]. Different bactericidal mechanisms of various nanosystems are primarily ascribed to their large surface area to the volume ratio [168] and unique physicochemical attributes. Briefly, in-depth studies of diverse nanomaterials are expected to strengthen our basic understanding on the antimicrobial mechanisms and fulfill the practical demands of the nanomaterials in the near future.
4. Conclusions
Based on the critical survey of the relevant literatures the following conclusions can be drawn:
- (i)Various methods have been developed to fabricate different nanomaterials-based antiviral and antibacterial coatings.
- (ii)The nanotechnology and nanomaterials became promising for customizing various properties of the materials desired for fighting the surface and airborne pathogens, bacteria and viruses.
- (iii)The manipulation of the materials' surface using various metal NPs spray or coatings is essential for the long-term disinfection in an easy, economic and yet safe manner.
- (iv)The TiO2 and ZnO NPs are the most common and efficient antimicrobial agents due to their bio-compatibility, non-toxicity, low cost, high chemical stability and environmental responsively. Thus, the TiO2 and ZnO nanocoatings have predominantly been used for the virucidal and bactericidal applications.
- (v)The NPs of Cu, Ag, Au, TiO2, SiO2 and ZnO have shown high antimicrobial activity. Therefore, these nanomaterials can be used to fight against the biodeterioration.
- (vi)It is asserted that diverse nanomaterials can be integrated into the existing building materials to improve their fundamental characteristics. In addition, the applications of different nanomaterials-based antiviral coatings may help to restrict or retard the spread of Covid-19.