Abstract
Graphene-based materials are capable of enhancing the refractometric response of prism- and optical fiber-based surface plasmon resonance (SPR) sensors; however, complicated multistep and time-consuming attaching processes could limit their practical applications. Herein, for the first time, we demonstrate the immobilization of graphene oxide (GO) submicrometric sheets onto the surface of a gold-coated single-mode fiber using a coating of fungal self-assembling proteins, the hydrophobins (HFBs), as an adhesive nanolayer. Hetero-core fiber tip SPR structures used in this study, consisting of a mirrored multimode–single-mode fiber structure coated with different thin layers (a chromium layer of 3 nm and a gold layer of 30 nm on top) exhibited a refractive index sensitivity (SRI) of 1842 nm RIU−1 (RIU: refractive index unit) at a refractive index (RI) of 1.36. Self-assembly of GO over the SPR fiber tip via HFB, offered an enhancement of up to 20% in the SRI. Moreover, this HFB-GO coating prevented degradation of the Al thin film mirror caused by corrosive salt-water solutions. The process is very simple, harmless, rapid (around 15 min) and scalable, as it is mostly based on one plasma treatment, which can be performed in large chambers and two dip coating steps, in liquid baths. All these features make the use of self-assembled bio/non-bio hybrid coating a green industrial method to improve the performance of SPR fiber biosensors, if compared with traditional chemical methods. Materials applied in this technology, fungal proteins and derivatives of graphite, are sustainable and largely available.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Refractometric techniques, a collection of optical instruments and devices to measure the refractive index (RI), are a powerful approach for real-time sensing in a myriad of processes related to medicine [1, 2], chemical industry [3–5], photonics [6, 7], food and beverages industry [8, 9], etc. Among them, optical fiber refractive index sensors (OFRIS) [10–18] represent an advantageous alternative for applications where the sensing point is difficult to reach, or the sample amount and/or the size of the sensing probe are a concern. Apart from their miniaturized nature, optical fibers (OF) combine telemetric capabilities, and immunity to electromagnetic radiation, along with high refractive index sensitivity (SRI) when exhibiting physical phenomena such as surface plasmon resonance (SPR).
The original structure of a fiber optic is designed to produce a strong confinement of light in the fiber core. However, generally, SPROF sensors involve modification of such an original structure to allow the interaction of a sensitive area exhibiting an evanescent field with the surrounding medium. This sensitive section is then coated with a thin film of noble metal [19–23], thereby resulting in an efficient plasmonic response, where the energy of the evanescent wave is coupled to the surface plasmon wave. The resulting plasmon wave propagates at the interface of the metal layer and the external medium. Hence, these coupling conditions are highly sensitive to the RI of the surrounding media.
Although SPROF sensors already exhibit a high SRI, it can be increased by depositing a thin layer of dielectric material with a high RI [24]. Recently, theoretical and experimental approaches demonstrated that hybrid structures composed by noble metals and overlayers of graphene derivatives, such as graphene oxide (GO), or reduced graphene oxide (rGO), produce an increment of the SRI of SPR-based sensors [25–36]. In fact, graphene-based coatings modify the propagation constant of surface plasmons and enhance the intensity of the field at the interface since their complex RI has a large real part in the visible range [37]. However, it is necessary to determine the optimum coating in terms of thickness and homogeneity of these graphene-based coatings since an increment of the number of graphene layers produces a nonuniform increment of the SRI in the SPR fiber optic sensor [38]. In this context, graphene monolayers [29, 30, 32, 33], polymer/rGO nanocomposites [31], Ag/rGO nanocomposites [34], rGO/chitosan/silica sol–gel composites [35], and Au/GO layers [36] have also been employed to this aim. However, the fabrication processes of these devices are generally complicated, multistep and time consuming.
Hydrophobins (HFBs) are small (a few nanometers in size in their monomeric soluble form) amphiphilic and adhesive fungal proteins that find biotechnological applications in sustainable surface modification and biomedicine [39]. Indeed, they have a large hydrophobic side exposed to the solvent, which is not usual in soluble globular proteins, and a more typical hydrophilic side. Because of the hydrophobic side, the HFBs self-assemble into oligomers in solution to minimize exposure to the solvent [40], and eventually adhere to surfaces forming different types of nanometric films.
Self-assembled coating based on Class I HFBs is particularly convenient, since they are more chemically robust than other protein-based films [41, 42]. Class I HFB films are composed of nanostructured aggregates, laterally packed amyloid-like fibers [43, 44]. Vmh2 is a class I HFB characterized by one large hydrophobic face, high propensity to adhere on surfaces, and low aqueous solubility [40, 45, 46], which we have previously utilized to immobilize peptides, proteins and nanomaterials, on different biomedical devices including biosensors [47–49].
The structures of the aggregates and thickness of the films vary with surface type, the HFB class (I or II) and the solvent conditions [43]. In addition, due to the rich variety of functional groups in proteins (side chains) and the high binding energy of peptide bonds (backbone) upon adsorption on graphene surface [50], they can easily adhere to chemically heterogeneous materials such as GO, which likewise is rich in both hydrophilic and hydrophobic surface areas [51].
Adhesion of the Vmh2 HFB layers on gold surface has been characterized by the authors both in dispersed nanoparticles [52], and on electrodes, the latter upon treatment with piranha solution [53]. Assuming a homogeneous protein nanolayer, the thickness on gold has been estimated to be ∼24 nm using spectroscopic ellipsometry. Fine structural analysis can be performed by AFM only on atomically flat surfaces. We have previously measured the thickness of Vmh2 rod-like nano-aggregates by AFM on silicon surface, and estimated them ∼3.5–4 nm [54], and the thickness of the Vmh2 plus a few layer GO coating on atomically flat mica [47]. Even though the high homogeneity of the film made it difficult to identify bare surface as high offset reference, the coating appeared thinner than 16 nm.
Herein, we developed an SPR-based OFRIS with enhanced SRI and resistance to corrosion via a hybrid HFB-GO coating, see figure 1. Firstly, the hetero-core fiber (HTCF) is fabricated by splicing a single-mode fiber (SMF) section between two multimode fibers (MMF) (figure 1(a)). Commercial and widely available SMF and MMF are used (figure 1(b)). The bare SMF section is then coated with a gold metal layer via thermal evaporation (figure 1(c)). A transversal cut is then performed to produce a mirror of aluminum via thermal evaporation in the tip of the SMF (figure 1(d)). Eventually, the GO decoration process was self-assembled layer-by-layer using Vmh2 as a primer for the immobilization of GO (figures 1(e)–(g)). The GO immobilization process is very straightforward and requires a few minutes (c.a. 15 min). It can be accomplished without the need of a controlled atmosphere or the use of corrosive and dangerous materials. Gold-coated hetero-core fibers (Au-HTCF) were decorated with HFB and GO submicrometric sheets at different concentrations, and then tested in a series of saline solutions. The SRI of our devices increased up to 20%, showing that the self-assembled coating technique is suitable to enhance the interaction of the original evanescent wave of the OFRIS with the external medium.
Figure 1. General schematic of the fabrication of our SPR-based optical fiber RI sensor decorated with HFB and GO submicrometric sheets. (a) Hetero-core fiber structure, (b) Cross-section of the multimode fiber (MMF) and single-mode fiber (SMF) showing the dimension of the core and cladding diameter. (c) SMF section of the hetero-core fiber coated with a gold metal layer, (d) Cleaved gold-coated hetero-core fiber and then coated with an Al mirror at its end face, (e) Hetero-core fiber coated with HFB, (f) Self-assembly of graphene oxide using HFB as a primer for GO immobilization. (g) Schematic representation of the resulting OFRIS.
Download figure:
Standard image High-resolution image2. Experimental section
2.1. Fabrication of the Au-HTCF
2.1.1. HTCF structures
A discussion on fundamental aspects of SPR-based OFRIS is included in the supporting information (SI). The hetero-core structure is simple to fabricate using standard OF, tools, and equipment existing in most OF laboratories. The mechanical strength of the structure is higher than other evanescent wave-based devices such as fiber tapers; therefore, they can be handled and manipulated without special care, facilitating the metal coating process and the use of the final device in real applications. Moreover, light excitation of plasmon waves in hetero-core structured fiber occurs at wavelengths in the visible and near infrared range, where a number of cost-effective power sources and detectors are commercially available.
The fabrication of the HTCF structure is straightforward, see figures 1 and S1 in the SI (available online at stacks.iop.org/JPPHOTON/3/034009/mmedia). Firstly, the lead-in MMF (62.5/125: core diameter/cladding diameter) was cleaved, ensuring that the length from the primary coating, used as a reference, was 10 mm to the end-face of the fiber tip (see figure S1(a), SI). Secondly, the MMF was spliced to a SMF (9/125) (see figure S1(b), SI). Then, the MMF-SMF fiber structure was placed over the fiber cleaver ensuring that the union between the MMF-SMF matched the cleaver blade. Thirdly, the fiber was moved 10 mm and the SMF was cleaved (figure S1(c), SI). Finally, the SMF end was spliced to another MMF (figure S1(d), SI). The final structure MMF-SMF-MMF is shown in figures 1(a) and (b).
To quantify the sensitivity of the HTCF structure to the changes of RI of the external medium, one of the fabricated HTCF was tested using a transmission experimental setup (see figures S2 and S3, SI). Consult the SI for more details.
2.1.2. Au-HTCF tips equipped with aluminum mirror in the end-face
The second stage of the fabrication process of the SPR-based OFRIS involved the deposition of a gold layer around the SMF perimeter using the thermal evaporation method. This process is complicated to achieve by thermal evaporation due to the cylindrical geometry of the OF. However, in a previous work, we demonstrated that the deposition of two gold layers, in opposite sides of the SMF section, is a feasible strategy to produce a semi-uniform gold layer with good SPR response [21]. The optimum length of the proposed fiber was also determined in a previous work [21]. Therefore, several HTCF structures were fabricated and fixed over a mechanical stand. This stand allowed to place the set of fibers inside the vacuum chamber and at a specific position with respect to the thermal evaporation source, see figure S4(a) (SI). In this context, c.a. 3 nm of Cr and c.a. 30 nm of Au were successively deposited over one side of the SMF section of the fibers, then the mechanical structure was rotated 180° to deposit an identical bilayer over the opposed side of the fibers surfaces, see figure 1(c). This is the final structure of the transmission SPR-based HTCF; however, for some applications is better to use the reflection SPR-based HTCF tip version. This device is simpler to manipulate, it can be introduced in a container to measure the RI of the liquid, and it is cleaned more easily. To obtain an SPR fiber tip, each HTCF was cleaved at the middle of the SMF section and mounted again in the mechanical stand to deposit an aluminum (Al) thin film of 40 nm over the end-face of the fibers as a mirror, see figure S4(b) (SI). Unlike the end-face of the SMF without a mirror only reflects back c.a. 4% of the incident light, attaching the Al thin film allows to reflect back more light into the MMF section and to the detector. A representation of the final Au-HTCF tip is shown in figure 1(d). By following this procedure, it was possible to coat up to 30 HTCF tips at the same time, thereby offering SPR response.
2.2. HFBs production
The class I HFB named Vmh2 was extracted from the surface of the mycelium of the edible fungus Pleurotus ostreatus, purified and dissolved in 60% ethanol (aqueous) at 0.4 mg ml−1, according to previously reported methods [45, 47]. HFB in 60% ethanol was stable for at least one year when stored at 4 °C.
2.3. Attaching the GO to the gold layer by means of HFB
Monolayer GO submicrometric sheets (average lateral size ∼500 nm) and carbon/oxygen weight ratio ∼1 were employed (characterization provided by the manufacturer: Angstron Materials, OH, USA). Figure 2 depicts the overall GO coating process by self-assembled bio/non-bio layer-by-layer hybrid coating onto the fiber tip following methods adapted from our previously reported study [47]. Briefly, the surface of Au-HTCF tips was negatively charged by an oxygen plasma treatment for 10 min (step 1) using a Femto low-pressure plasma system (Diener; Ebhausen, Germany) filled with extra dry oxygen (99.5% purity) and a gas pressure of 0.6 bar. Then, using the HFB concentration of 50 ng μl−1 recommended [47], the SPR fiber tips were soaked in HFB solution for 2 min (step 2). Later, they were removed from the HFB solution, rinsed three-times with ethanol (60%), and dried with nitrogen; the structure of the device is represented in figure 1(e). Finally, each fiber tip was immersed for 10 min in different concentrations of GO (50, 100, 200, 400, 800 and 1600 µg ml−1) to allow adhesion of GO sheets to the HFB layer, rinsed three-times with ultrapure water and dried with nitrogen (steps 3 and 4). A representation of the final Au-HTCF tip decorated with HFB and GO sheet is shown in figure 1(f). For simplicity, the Au-HTCF tips fabricated were labeled according to the table 1.
Figure 2. Self-assembly process to immobilize GO sheets over the SPR fiber tip utilizing HFB as a linking intermediate layer.
Download figure:
Standard image High-resolution imageTable 1. Labeling of the fiber tips fabricated.
| Tip | Plasma treatment and HFB | GO concentration (μg ml−1) |
|---|---|---|
| Au-HTCF | Without | 0 |
| Au-HTCF-0 | With | 0 |
| Au-HTCF-I | With | 50 |
| Au-HTCF-II | With | 100 |
| Au-HTCF-III | With | 200 |
| Au-HTCF-IV | With | 400 |
| Au-HTCF-V | With | 1600 |
The SPR spectra of the Au-HTCF-I, II, III, IV, V were measured, then, the RI sensitivity ( ) of each sample was calculated. The sensitivity enhancement percentage of the Au-HTCF-I, II, III, IV, V, due to the HFB + GO coating, was obtained by using the following relationship and the sensitivity of each Au-HTCF sample before the functionalization as a reference (
) of each sample was calculated. The sensitivity enhancement percentage of the Au-HTCF-I, II, III, IV, V, due to the HFB + GO coating, was obtained by using the following relationship and the sensitivity of each Au-HTCF sample before the functionalization as a reference ( ):
):
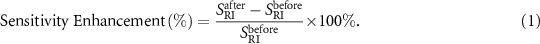
Sensitivity enhancement on sample Au-HTCF-0 was not reported due to the lack of reproducibility. Additionally, Au HTCF tips decorated with HFB and GO sheets were characterized via Raman spectroscopy and other pictures were obtained using a VHX-5000 digital microscope (Keyence; Osaka, Japan).
2.4. Raman and SEM characterization
Raman spectra were recorded using an inVia Raman module (Renishaw; Wotton-under-Edge, UK), which is coupled on a Leica DM 2500M microscope in a vertical configuration. A 50× objective (NA = 0.75) was employed in all the experiments. The excitation line at 514 nm was provided by an argon laser (0.8 mW). In all the experiments, the laser power was at 5% (size of the spot ≈ 1.2 μm) and a static scan was performed during 1 s, with 35 accumulations. At least three spectra were acquired in random positions of the studied samples.
SEM analysis was performed using a field emission of high resolution, JSM-7800F (JEOL; Akishima, Tokyo, Japan), acceleration voltage of 1 kV.
3. Results and discussion
3.1. Characterization of Au-HTCF tips to RI
The experimental setup depicted in figure S5 (SI) was used to measure the changes of the reflected spectrum of the Au-HTCF tips, which are not decorated with HFB and GO sheets, see figure 1(d). To characterize the fiber tips a series of saline solutions were prepared, at a NaCl concentration of 0%, 12%, 16%, 20%, 26%, and 30%, corresponding to a RI of 1.3325, 1.3515, 1.3575, 1.3625, 1.3700 and 1.3745, respectively. The RI of the saline solutions was measured with a commercial Abbe refractometer (model WY1A, BAUSH L). As followed, an Au-HTCF tip was spliced to the output port of the MMF coupler, as shown in figure S5 (SI) and the reflected spectrum was recorded.
The spectrum, when the external medium was air with a RI of 1.00032, was used as the reference spectrum. All the spectra measured were divided by the reference spectrum to obtain the reflected SPR spectrum. When the fiber tip was immersed in milliQ water the SPR spectrum exhibited a dip, the wavelength of the dip minimum, also known as the resonance wavelength, was 580 nm as can be seen in the red plot of figure 3(a). When the fiber tip was immersed into the 12% solution of NaCl, the SPR spectrum exhibited a redshift. After each test, the fiber tip was cleaned and dried before immersing it into the next solution. The SPR dip shifted to longer wavelengths when the fiber tip was immersed into the 16% solution of NaCl. Figure 3(a) shows the reflected spectra of these two concentrations in the dark blue and purple plots, respectively. Interestingly, when the fiber was immersed in 20% solution of NaCl, the level of the reflected signal decreased and the characteristic SPR dip disappeared, as can be seen in the green plot of figure 3(a). This fiber tip was substituted by another one and the procedure was repeated, we observed the same behavior. We concluded that the drastic drop in the reflectivity of the aluminum thin film mirror (without the proposed GO coating) was due to the corrosive action of the NaCl.
Figure 3. Reflected SPR spectrum obtained when the Au-HTCF tip not decorated with HFB and GO sheets were immersed into (a) saline and (b) sucrose solutions at different concentrations. (c) Displacement of the SPR wavelength dips as a function of RI of the external medium, the inset shows the FOM (figure of merit) of the SPR fiber tip.
Download figure:
Standard image High-resolution imageThe RI characterization of the Au-HTCF tips was completed using solutions of water and sucrose. Their concentration was calculated to have the same RI to that of the solutions prepared with NaCl. The results of the experiments with milliQ water and sucrose solutions showed that the SPR spectra, see figure 3(b), redshifted as the RI of the solutions increased. Figure 3(c) indicated the displacements of the resonance wavelength (ΔλResonance) as a function of the RI of the solutions. The resonance wavelength is defined as the point where the power of the reflected light is minimum, and the shifting of the resonance wavelength for each solution with respect to the resonance wavelength of the first solution is known as ΔλResonance. The behavior of the resonance wavelength shift is represented by a second order polynomial fit showing a directly proportional relationship with the RI of the external medium. Besides, the FOM (figure of merit) of the device defined as the ratio of ΔλResonance over the FWHM (full width at the half minimum dip) of the SPR dip, is almost constant, see inset of figure 3(c). This is because the FWHM of the dip also increases as the RI of the external medium does. Although the water in sucrose solutions could produce metal corrosion, we did not observe any unusual behavior on the response of the Au-HTCF tip that could indicate degradation of the metal thin films attached to the fiber, see figure 3(b). The RI sensitivity of each Au-HTCF before coating with the HFB + GO was calculated ( ).
).
3.2. The refractometric response of the resulting OFRIS
The refractometric response of HTCF tips coated with 30 nm of gold and decorated with HFB and different concentrations of GO was analyzed using the experimental set up shown in figure S5 (SI). Fibers were cleaned with ultrapure water and dried with compressed air before the characterization. Then, the fiber under test was successively immersed in saline solutions at different concentrations of NaCl (0%, 12%, 16%, 20%, 26%, and 30% w/v). After that, the respective fibers were cleaned with ultrapure water and dried with air. Figure 4(a) shows the SPR spectra of the Au-HTCF-IV. Importantly, the Al mirror deposited over the end-face of the SPR fiber tips was not affected by these saline solutions, although, the full set of experiment measurements lasted 1 h. In contrast, as previously shown, see figure 3(a), the Au-HTCF tip not decorated with HFB and GO sheets was stable only for three measurements using relatively low concentrations of NaCl (0%, 12% and 16% w/v). Hence, we discovered that the employed biohybrid coating protected the fiber tip from corrosion. By tracking down the wavelength of the SPR dip, we determined its relationship with the RI of the external medium for each GO concentration, as shown in figures 4(b) and S6 (SI). We noticed that the sensitivity of Au-HTCF-I tip decreased at low RI, with respect to the Au-HTCF tip. A Raman analysis performed over the Au-HTCF-I did not show deposited GO due to the relatively low GO concentration, see figure S7(a) in the SI. Therefore, most of the exposed fiber surface was coated with HFB only, which is known to change the wettability of the surface, making the sensor hydrophobic, which affects the surface interaction with the aqueous solvent. As the concentration of GO increased, the sensitivity of the device also increased, reaching a maximum sensitivity when the concentration of GO was 400 μg ml−1. At this concentration, the fiber response reported a 20% enhancement as shown in figure 4(c). However, we noticed that the sensitivity of the device started to decrease again at a GO concentration of 1600 μg ml−1.
Figure 4. (a) Reflected spectra Au-HTCF-IV tip decorated with HFB and GO sheets when it was immersed in solutions with different NaCl concentration, spectra are redshifted due to increment of the refractive index of the solutions and (b) the relationship between wavelength displacement of the SPR dip and the RI of the external medium. (c) Sensitivity enhancement percentage of the Au-HTCF-I, II, III, IV, V owing to the HFB + GO coating.
Download figure:
Standard image High-resolution imageThe aforementioned sensitivity enhancement is in good agreement with the Raman analysis shown in figure S7(a) in the SI, where the maximum intensity of the corresponding Raman fingerprint was also observed in Au-HTCF-IV. We hypothesize that this behavior of the GO concentration at 400 μg ml−1 provided the optimal conditions of mass transport in the corresponding decoration process, thereby leading to the most homogenous coating observed in this series of experiments, which is supported by the corresponding strong intensity of the characteristic Raman signature of GO (D and G bands).
Due to the high roughness of gold surface at the atomic level, we performed SEM analysis of the coating instead of conventional AFM, which roughly indicated a homogeneous distribution of flakes on the surfaces in the optimal conditions for sensing (400 µg ml−1 GO), and probably lower surface coverage at lower concentration. As observed by SEM imaging, lower or higher GO concentrations did not yield such a homogeneous coating, see figure 5 (this is confirmed when figure 5(d) is compared with the rest of the panels).
Figure 5. SEM micrographs of the surface of the fibers coated at different GO concentrations; (a) 50, (b) 100, (c) 200, (d) 400, (e) 800, (f) 1600 µg ml−1. The scale bars represent 1 μm.
Download figure:
Standard image High-resolution imageAn important feature of a RI sensor is its response to ambient temperature changes. To test this, the Au-HTCF-IV tip was immersed in ultrapure water. For this experiment, the solution was heated and the spectrum was recorded at 25 °C, 30 °C, 35 °C, 40 °C, 45 °C and 50 °C. Figure 6(a) shows the SPR curve of the Au-HTCF-IV tip. We noticed a blue-shift of the resonance dip as the temperature increased. The wavelength shift of the dip is shown in the inset graph. We attributed this displacement to the change of the RI of the solution due to temperature increment. This change in the RI is regulated by the thermo-optic coefficient (TOC, RIU °C−1) of the solution under test. Generally, the TOC of aqueous solutions is negative [21], and thus the RI decreases as the temperature increases. We observed that the resonance dip shifted −0.1508 nm °C−1, indicating that this sensitivity is smaller, thus advantageous, when compared with that previously reported using a transmission scheme [21].
Figure 6. (a) SPR spectrum of Au-HTCF-IV tip when it was immersed in ultrapure water, at different temperatures. (b) Stability of the Au-HTCF-IV tip over a three-month period.
Download figure:
Standard image High-resolution imageThe stability of the sensor response during a time lapse was also tested. The Au-HTCF-IV tip was properly cleaned, dried, and then fixed to a mechanical mount without any special protection. Then, the reflected spectrum was recorded during a period of three months. Measurements were made at a constant temperature of 25 °C. Figure 6(b) shows four spectra obtained when the fiber tip was immersed in a solution with a NaCl concentration of 30% exhibiting a RI of 1.3745 (previously measured with an Abbe refractometer). As a result, no significant change was observed in the response of the device during the first month. However, in the second and third month we noticed a 6 nm blue-shift of the spectrum dip for every month, corresponding to a RI deviation of −0.0011 of the substance. Our device was not drastically affected in time, since the shape and the depth of the SPR curve were not affected, but a stability study for the RI sensor is important in order to estimate their long-term response and to identify the mechanism that can affect this.
4. Conclusions
We studied a straightforward, harmless and fast method to immobilize GO over the surface of gold-coated fiber tips using fungal HFB as a nanoadhesive. In particular, Au-HTCF were fabricated and decorated with HFB and then GO, self-assembled layer-by-layer. GO improved the refractometric sensitivity of the SPR fiber tips. Particularly, using a GO concentration of 400 μg ml−1 in the coating step, sensitivity was enhanced up to 20%. Protein based layers are known to not have excellent chemical stability. However, our experimental evidence related to the stability of the proposed HFB-GO-coated fiber revealed that the self-assembled bio/non-bio hybrid coating was stable for at least three months at room temperature. Moreover, the HFB-GO coating applied on the Al metal layer at the fiber end-face tip prevented corrosion provoked by concentrated saline solutions upon repeated measurements. Indeed, the particular protein used in this study (the class I HFB, namely Vmh2 from Pleurotus ostreatus) is known to self-assemble and aggregate into homogeneous and chemically robust films, and in addition, with GO on top, the HFB layer is protected and safe. In fact, the physicochemical properties of GO (its wide planar, impermeable and heterogeneous surface) enable wide and numerous contacts with the protein-coated surface (and therefore high energy of adhesion), and at the same time, protect the protein layer from corrosion. This research will pave the way to the development of fiber RI sensors that require high sensitivity, for example in label-free (bio)sensing applications, and resistance for real-world device fabrication. Given the usage of sustainable materials (fungal proteins and graphite derivatives), and scalable processes (plasma surface treatment, and dip coating), this process is not only easy but also industrially applicable.
Acknowledgments
The authors are grateful to the Consejo Nacional de Ciencia y Tecnología (Mexico) for financial support through the projects CB 257046 and LN 293442. Monserrat del Carmen Alonso-Murias would like to thank CONACyT for their support through a PhD scholarship.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).







