Abstract
Cancer therapy might benefit significantly from nanotechnology. These nano-drug delivery systems (NDDS) have been established to improve the therapeutic benefits of anticancer medications by enhancing their bioavailability, degradation, and biocompatibility. One of the most promising NDDS for cancer therapy is high-performance hydroxyapatite (HA) nanoparticles, which have many advantages. The metabolite marizomib inhibits tumor cell growth and progression, functioning as a biochemical inhibitor in many malignancies. However, this substance's low bioavailability is the most significant problem with its use. In this work, a pH-sensitive biopolymer was employed to encapsulate HA nanoparticles with chitosan to increase marizomib's (MARI) efficacy and bioavailability. The sol-gel process was used to fabricate HA nanoparticles for this purpose. It was then coated with chitosan before encasing the marizomib drug in the nanocarrier, which was done under controlled circumstances. The newly fabricated nanoparticles effectively kill ovarian A2780 cancer cells and induce apoptosis. The morphological examination of the cancer cells was examined by AO/EB and DAPI staining methods. Further, the cell uptake was measured by the flow cytometry methods, and the result shows the nanoparticles were effectively uptake the cancer cells under different incubation times. In principle, nanoparticles have great potential for future pre-clinical applications in treating ovarian cancer cells and suppressing other types of tumors.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cancer is the most lethal human disease. By 2025, GLOBOCAN 2021 predicted that there would be 19.3 million new cancer cases per year globally, up from 14.1 million in 2021 [1]. Chemotherapy, surgery, radiation, and immunotherapy are the four most common forms of cancer treatment performed worldwide [2]. Traditional cancer treatments have many drawbacks, including high toxicity, poor oral bioavailability, lack of water solubility, low therapeutic indices, inconsistent circulation, non-specific biodistribution, and administration of anticancer drugs to both normal and malignant cells [3–5]. As a result of these high mortality and morbidity rates, new, safe, effective therapies are urgently required [6–8]. Polymeric nanoparticles (PNPs) are colloidal particles with less than one-micron diameter. Adsorbed, encapsulated, or conjugated anticancer agents may be found within or on the surface of PNPs [9–11]. PNPs loaded with drugs are employed in a targeted delivery system to ensure anticancer therapies reach their intended sites [12]. Targeted drug delivery system (TDDS) development is based on the rational design of polymer (composition, solubility; crystallinity; molecular weight; backbone stability; hydrophobicity; and polydispersity) customized to a specific payload (molecular weight and charge of drug) [13–15].
A highly effective proteasome inhibitor, Marizomib (NPI-0052), was identified while screening extracts from the recently discovered marine actinomycete Salinispora tropica [16]. In the previous investigation, the CT-L activity of human and rabbit 20S proteasomes was inhibited by marizomib (IC50 4nM). Additionally, Marizomib also inhibits the C-L activity of 20S proteasomes at a comparable or lower efficiency compared to the T-L action. Anti-inflammatory cytokines, including TNF-a, IL-1, and IL-6, have been demonstrated to be inhibited by marizomib [17–19]. Adhesion molecules like ICAM-1 and VEGF have also been proven to be inhibited by marizomib [20]. Because Marizomib is not based on a peptide bond, its structure and characteristics are quite distinct from those of other proteasome inhibitors now used in clinical practice [21]. The toxicity and effectiveness of marizomib seem to vary from those of bortezomib because of its extended duration and distinct pattern of proteasome inhibition. As a single drug, marizomib has been explored in various clinical studies [22]. Data from pharmacodynamic studies showed that marizomib is a potent inhibitor of all three proteasomal functions and that inhibition is dosage, cycle, and schedule dependent [23]. According to pharmacokinetic studies, the half-life is brief, clearance is quick, and the drug is widely distributed. There is no buildup or activation of metabolism as the dose-dependent exposure rises linearly [24]. Marizomib is typically well-tolerated up to an MTD of 0.6–0.7 mg m−2 when given on day 1, 8, and 15 schedules [25]. Peripheral neuropathy, neutropenia, and thrombocytopenia are not frequent side effects of bortezomib. In patients with myeloma who had previously been treated with bortezomib, tumor evaluations have shown that marizomib had antitumor efficacy [26].
Biocompatibility, bioavailability, and osteoconductivity are only hydroxyapatite's many essential features [27–31]. It is also non-toxic, non-inflammatory, and non-immunogenic, and its ultrafine structure resembles that of naturally-occurring hydroxyapatite [32–34]. Antibiotic (ciprofloxacin) and anticancer drug delivery systems based on calcium (HA) have previously been described in the literature [35]. Hepatocellular carcinomas, colon carcinomas, and stomach carcinoma are especially sensitive to the cytotoxic effects of HA. Nanocrystals with diverse morphologies and sizes may be formed utilizing a chemical precipitation approach that includes the use of a 'capping agent.' A polymer coating may also be applied on the surface of the HA nanoparticles to increase their surface functionality and mechanical properties [36–38]. HA may be modified to create different-sized nanocomposite materials with improved mechanical characteristics by using Chitosan (Cht). Chemical changes, biocompatibility, biodegradability, and minimal cytotoxicity make chitosan an ideal carrier for pharmaceuticals in controlled release [39–41]. Because of its amphiphilic nature, chitosan has been shown to enhance paclitaxel's sluggish removal and prolonged release [42]. The incorporation of drugs and ligands and the reduction of cytotoxicity are both dependent on nanoparticle size, surface charge, and material composition [43]. The electrostatic interaction between the positively charged chitosan nanocarriers and the negatively charged cancer cell membranes improves absorption efficiency. Previous research has shown that hydroxyapatite/chitosan composites may deliver antibiotics [44].
A new drug delivery strategy for marizomib using nanocarriers of chitosan and HA was the primary goal of this work. First, hydroxyapatite nanostructures were fabricated and analyzed to attain this aim. HA particles were subsequently coated with chitosan biopolymer, which is pH-sensitive. Marizomib was then administered to the collected sample. FIR was utilized to verify that chitosan encircled and bound to HA particles and absorbed the drug. In contrast, the nanocarrier-encapsulation efficiency and drug loading capacity may be determined using the spectrophotometric approach, as well as the release profile of the drug in an acidic and neutralizing medium. Fluorescence microscopy and tetrazolium experiment were used to measure cytotoxicity and the activation of apoptosis in A2780 cancer cells. Imaging and flow cytometry was used to investigate how nano-drugs penetrate tumor cells.
2. Experimental section
2.1. Materials
Chitosan 90% (deacetylation), dimethyl sulfoxide (DMSO), ammonium phosphate, calcium nitrate, marizomib were purchased from Dalian Meilun Biotechnology, Co., Ltd Methyl-thiazol-tetrazolium (MTT), powder propidium iodide, trypan blue colour, RPMI-1640 culture medium, phosphate-buffered saline (PBS), amphotericin B powder, and dialysis bag were purchased from Shanghai Sangon Biological Engineering Technology & Services Co., Ltd (Shanghai, China). Trypsin-EDTA enzyme (0.25%), fetal bovine serum (FBS), acridine orange and ethidium bromide, penicillin G antibiotic, and streptomycin antibiotics were purchased from Abcam (Cambridge, MA, USA). Water was obtained from a Milli-Q Integral Water Purification System (18.2 MΩ cm−1).
2.2. Characterization
Transmission electron microscopy (TEM) images, high-resolution TEM (HRTEM) images were taken at 300 kV with an FEI Tecnai G2 F30 microscope. Field-emission scanning electron microscopy (SEM, Bruker Gemini500) was applied to characterize the morphology and structure of the as-prepared products. FTIR spectra were recorded on PerkinElmer Spectrum Two Pharmaceutical System. The hydrodynamic diameter was measured using a Zeta Sizer Nano ZS90 (Malvern Instruments Ltd, Worcestershire, U.K.). A Zeiss LSM 800 laser scanning confocal microscope (Zeiss, Germany) was used for cell imaging. A CytoFLEX flow cytometer (Beckman coulter, USA) was used for cytofluorimetric analysis.
2.3. Preparation of n-HA
The sol-gel technique was used to synthesize HA with a Ca/P stoichiometric ratio of 1.67. To get the reaction performed [45–47], a combination of water and ethanol (1:12 by volume) was poured into the reaction flask, which contained around 50 mmol of calcium nitrate tetrahydrate (CNTH). Slow addition of (14.5 M) ammonia solution was used to keep the pH of the solution in the range of 10–12. Reactions were carried out for 4 h with intensive 400-rpm stirring of the diammonium hydroxide (DAHP) solution in water, which contained 30mmol of DAHP. Using an electric heater, the electric heater reaction temperature was retained at 45 °C. The reaction products were collected and rinsed with distilled water several times using a filter paper before being dried in an oven at 40 °C for 14 h. After that, the product was dried in a vacuum oven at 60 °C for 2 h than at 80 °C for 18 h until it was entirely dry. Once it reached 500 °C, it was held for an hour at an oven heating rate of 10 °C min−1.
2.4. Preparation of M-CNH
0.5, 1, 1.5, 2, and 2.5 mg of marizomib were diluted in 10 ml of ethanol in separate glass containers. Sonication was then used to introduce 10 mg of n-HA nanocarrier to each container. For 24 h, the reaction samples were kept in the dark and at 4 °C on a stirrer. Next, 20 ml of chitosan solution (w/v) with citric acid (1.5 w/w) was added to the reaction and stirred at room temperature for 12 h. After drying using a freeze drier, the samples were collected and centrifuged for 20 min at 10,000 rpm. They were then cleaned three times with water and ethanol. This research used chitosan-coated HA nanocarriers (CNH) and marizomib-coated HA nanocarriers (M-CNH).
2.5. Measurement of the encapsulation efficiency (EE) and drug-loading content (DLC) of marizomib
To determine the quantity of DLC and EE in the various drug complexes (each having varying concentrations of marizomib), 1 ml of ethanol was dissolved in 10 mg of each compound. After that, each sample was exposed to 15 min of shaking and sonication to extract the drug fully from the nanocarrier. A spectrophotometer was used to measure light absorption intensity at the drug's maximal absorption point of 425 nm. It was used to calculate the standard curve's line equation for the observed results. Calculations of DLC and EE in nanocarriers were made using equations (1) and (2).
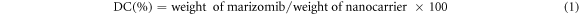
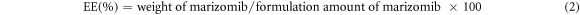
2.6. Analysis of drug release
A dialysis bag with a 12 kDa molecular weight limit was used to test the drug release profile by dissolving equal volumes of lyophilized M-CNH (10 mg) in 0.1 M PBS. The samples were then placed in an incubator at 37 °C and incubated at pH values between 5 and 7.4 in separate 20 ml PBS solutions. After a while, random samples were taken from each setting under study. The duration of this procedure was up to 96 h. Spectrophotometry was used to measure the concentration of marizomib liberated from the buffer solution. The proportion of marizomib released from the nanocarrier relative to its loaded value was shown as a function of time in a graph [48].
2.7. Cell culture conditions
A2780 (as cancer group) and NIH-3T3 (as a normal group) cells were purchased from KeyGen Biotech Co., Ltd (Nanjing, China) and cultured in RPMI-1640 medium supplemented with 10% (v/v) FBS, 0.03% L-glutamine, and 1% penicillin-streptomycin within a humidified atmosphere containing 5% CO2 at 37 °C. The cell culture medium was replaced every two days.
2.8. In vitro cytotoxicity assay
96-well microplate wells were filled with 6 × 103 cells, and the cells were allowed to attach and grow in a 37 °C cell culture incubator with 5% CO2 until they reached the correct density and morphology. Extracted supernatant and treated drug values were added to the testing wells after incubation was completed. The plate was pre-incubated according to protocol. Three different concentrations were tested. Next, it was necessary to remove the top layer of culture media from each well and then add to each well 25 μl of PBS buffer that included 0.50 μg ml−1 of MTT reagent. During this time, the enzyme succinate dehydrogenase, which is part of the respiratory cycle of mitochondria, revives the tetrazolium salt and forms aqueous crystals of formazan, which are readily visible under a microscope. Biochemical activity in live cells affects the color intensity of the dye. Before colorimetry can be performed, the crystals of formazan blue must be dissolved in an organic solvent such as DMSO. Once the incubation period was completed, a 150 μl volume of deionized water (DMSO) was added to each well. At a wavelength of 570 nm, the solution's optical absorption was observed. After measuring the absorption ratio of control groups, we determined cytotoxicity levels. MTT assay was used for 48 h to test the biocompatibility of CNH and n-HA nanocarriers. Concentrations of 0–600 μg ml−1 were used. These cell lines were tested at 24 and 48 h for cytotoxicity of the M-CNH formulation and the free drug at various doses (0–30 μg ml−1). Free marizomib therapy was carried out by dissolving the drug in organic methanol solvent, with a maximum concentration of 0.1% by volume being used [49–52].
2.9. Cell morphological analysis
Cells from A2780 or NIH-3T3 were treated for 24 h with a concentration of 13 μg ml−1 (equivalent to the M-CNH IC50 after 24 h) of MARI and M-CNH to determine morphological alterations. A 1-minute DAPI staining followed a 20-minute methanol fixation step to reveal the cells' identities. After that, the wells were examined with fluorescence microscopy and thrice rinsed with cold PBS (Olympus, Japan). There were no experimental samples in this investigation. Thus untreated cells were used as controls. The treated cells were harvested and centrifuged at 1500 rpm for five minutes before being suspended in 500 microliters of media for AO/EB staining, revealing whether apoptosis has been triggered in A2780 or NIH-3T3 cells after exposure to 13 μg ml−1 of MARI and M-CNH for 24 h. With 10 μl cell suspension, 5 μl of the solution, and 5 μl of the antibody, we conducted AO/EB staining [53–55]. After five minutes, the mixture was examined using fluorescence microscopy (Olympus, Japan). The fraction of living cells was determined by applying the following formula:
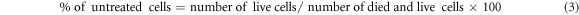
2.10. Evaluation of cellular uptake and internalization of M-CNH by flow cytometry
Marizomib is incorporated into DiI, and when using flow cytometry of marizomib, it is possible to determine how much of the nano-drug is absorbed by cancer cells. Cells were cultivated in each well of the plate for 24 h and incubated at 37 °C for 24 h for use in the flow cytometric analysis. To test the effect of the M-CNH nanodrug and free marizomib, the cell media was replaced with a concentration of 13 μg ml−1 M−1-CNH nanodrug in each well of RPMI-1640 culture medium and incubated for 2, 4, or 8 h. Afterward, the cell culture medium was withdrawn, and the cell layer washed three times with PBS to remove nano-drugs from the cell layer. The BD FACSCalibur Flow Cytometry System was eventually used to identify the analysis (BD Biosciences, USA). As a control, cells were treated with drug-free nanocarriers [56].
3. Results and discussion
3.1. Preparation and characterization of M-CNH
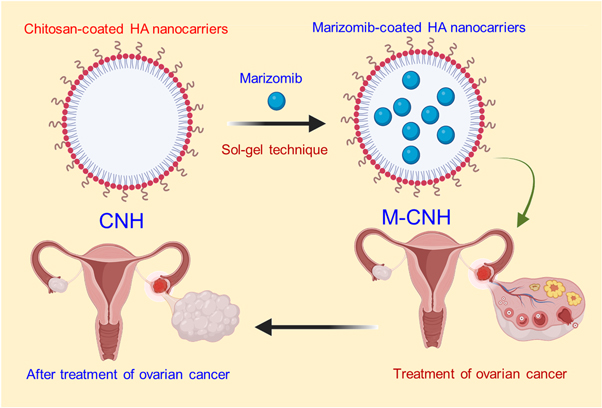
The drug marizomib was synthesized as chitosan-coated HA nanoparticles in this study. The sol-gel method was first used to fabricate HA nanoparticles to achieve this objective. These nanoparticles were then tested in vitro for their capability to reduce the improvement of ovarian cancer cells (Scheme
Scheme 1. Graphical representation of chitosan-coated HA nanoparticles encapsulated the marizomib drug by sol-gel method to treat ovarian cancer cells.
Download figure:
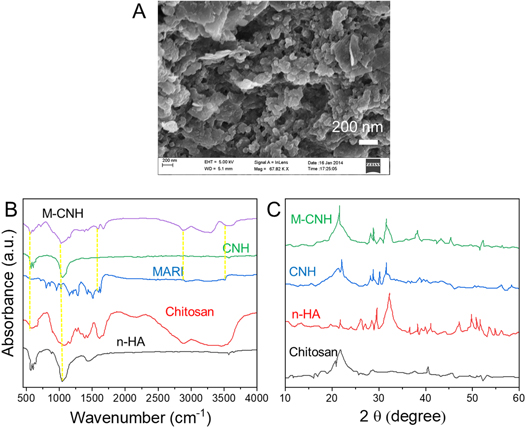
Standard image High-resolution imageFigure 1. SEM image of uniform M-CNH nanoparticles. (B) FTIR spectra of n-HA, chitosan, free MARI, CNH, and M-CNH. (C) The XRD patterns of n-HA, chitosan, CNH, and M-CNH.
Download figure:
Standard image High-resolution imageThe XRD measurements confirmed the nanoparticles' size. XRD patterns of chitosan, n-HA, CNH, and M-CNH particles are shown in figure 1(C). The n-HA powder has been subjected to XRD analysis, indicating a weakly crystalline substance. Chitosan's pattern has no discernible diffraction peaks. Although their strength is decreased, n-HA peaks can also be shown in the XRD patterns of CNH and MARI-loaded samples. Furthermore, the n-HA coated with chitosan has decreased or omitted the diffraction peaks associated with the (222), (213), (321), (410), and (402) planes. The associated XRD pattern showed no further peaks signifying the MARI drug. Marizomib-coated HA nanoparticles containing chitosan were established in this study. These nanoparticles were then subjected to in vitro analysis and drug release behaviour to see whether they might inhibit the development of ovarian cancer cells. It was shown that the chitosan molecules linked to the particle surface interfered with the dissolution re-precipitation process, reducing the crystallinity of particles.
3.2. Calculation of drug loading in nanocarriers
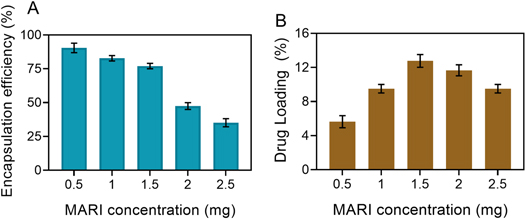
Marizomib-nanocarrier solution complex (M-CNH) was passed through 0.22 m syringe filters to remove the insoluble marizomib that was not loaded into the nanocarrier. Then, each drug combination was freeze-dried to fabricated powder samples. For a 15% marizomib: nanocarrier (w/w) ratio, the average EE and drug loading rate were 75.1% 4.36 and 12.46% 0.99, respectively, using equations (1) and (2). (figure 2(A)). Additionally, the ideal sample of 15% marizomib: nanocarriers (w/w) was utilized to validate the encapsulation of marizomib in nanocarriers by the FTIR technique (figure 2(B)). However, Sahu et al found the greatest marizomib efficacy in mPEG-PA carriers, only 31%. Still, in this study, the highest marizomib efficacy was shown in M-CNH nanocarriers at more than 2.95 times the ratio of 15% marizomib to nanocarriers, making this study even more important.
Figure 2. Encapsulation efficiency (EE) and marizomib's drug-loading content (DLC) in CNH nanocarrier. Data were represented as mean ± SD (n = 3).
Download figure:
Standard image High-resolution image3.3. Results of drug release profile
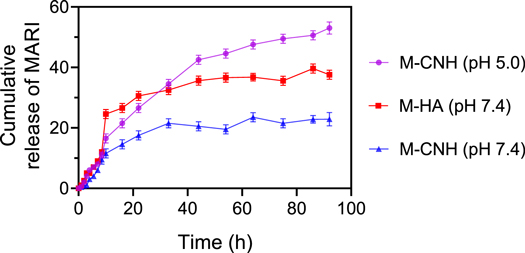
A buffer media with various pH levels was used to study the marizomib release kinetics from CNH nanocarriers (figure 3). This was accomplished by sampling different drug formulations in acidic and neutral environments, determining the drug release rate, and then using a spectrophotometer to determine the quantity of drug released after centrifugation. The duration of this procedure was up to 96 h. According to the study findings, the release of marizomib from CNH nanocarriers is slow and stable. The drug release rate from the nanocarrier was 38.22 ± 1.21% and 19 ± 2.69% after 44 h in the neutral and acidic environments, respectively, since the pH of the environment facilitates the drug release process. This might be evidence that CNH nanocarriers are pH-sensitive.
Figure 3. MARI release profile from the pure n-HA at pH = 7.4 and CNH under pH 7.4 and 5.0 for 96 h. Data were represented as mean ± SD (n = 3).
Download figure:
Standard image High-resolution imageHowever, since n-HA are not regarded as a pH-responsive system by itself, only the behaviour of this system in a PBS buffer solution with a pH of 7.4 was examined in this work. Comparing n-HA and CNH drug release profiles at various pH levels, CNH nanocarriers have lower release rates and better stability than n-HA. Finally, drug release rates from CNH in an acidic environment and n-HA and CNH were respectively 50.65 ± 0.99%, 37.40 ± 1.63% and 20.88 ± 3.63% in 96 h. According to the findings, chitosan's free amino and hydroxyl groups may ionize to ammonium and hydronium ions when exposed to H+ ions in an acidic environment, leading to swelling due to ion repulsion [57]. The protective coating around the n-HA channels opens due to this occurrence, allowing the drug to be released into the medium. Because H+ ion concentration falls with increasing pH, ammonium and hydronium ions reduce, resulting in decreased water absorption [58]. Additionally, the chitosan polymer chains collect around the n-HA molecule, making it difficult for the drug to diffuse freely. The release rate of marizomib from nanocarriers in PBS with pH = 7.4 is around 28% in the 1 h, and after 7 h, 50% of the drug is released. Using this method has resulted in many issues, including the drug being delivered non-targeted rather than selectively and a high drug release rate [59]. The release of marizomib from the CNH nanocarrier is gradual and sluggish in this research, as previously noted, and has excellent stability rather. Adding to this, reducing the pH of the surrounding environment helps the nanocarrier release the drug.
3.4. Evaluation of cytotoxicity by MTT assay
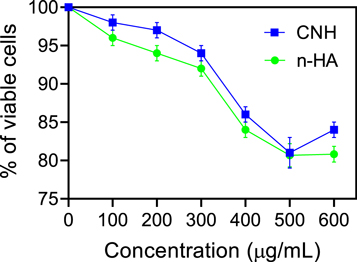
The biocompatibility of the nanocarriers utilized must be assessed before the performance of M-CNH nano drugs may be examined in live cells. Normal NIH-3T3 was treated with each prepared concentration of CNH and n-HA nanocarriers for 48 h. In the end, the cytotoxicity of each sample was tested against that of untreated cells using the MTT assay. Using CNH nanocarriers, this research found that the survival ratio of normal cells was over 93.48 ± 2.54%. Even while n-HA with a comparable concentration may help these cells survive for 90.69% of the time, the survival ratio of cells is only approximately 91.97 ± 4.85%. (figure 4). The findings suggest that a chitosan coating around n-HA may increase the biocompatibility of particles.
Figure 4. Examination of in vitro cytotoxicity of CNH and n-HA nanocarriers using MTT assay on normal NIH-3T3 cells. Data were represented as mean ± SD (n = 3).
Download figure:
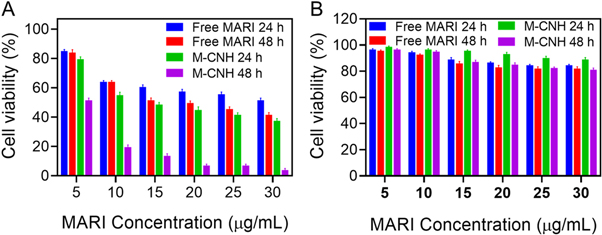
Standard image High-resolution imageAfter being treated with free marizomib and M-CNH at various doses (0–30 μg ml−1) for 24 and 48 h, the MTT assay was used to determine the survival rate of healthy cells and cancer cell novel. Free marizomib was dissolved in a 0.1% methanol solvent in this investigation. While the free M-CNH did not affect the survival of A2780 cells, the addition of M-CNH drastically decreased their survival rate. For free marizomib and M-CNH, the IC50 concentrations were 24.23 ± 2.74 μg ml−1 in only 24 h and 13.01 ± 1.66 μg ml−1 in just 48 h (figure 5(A)). On normal cells (NIH-3T3), free marizomib and M-CNH at various doses (0–30 g ml−1) were tested at 24 and 48 h using an MTT assay. NIH-3T3 cells were shown to have a greater survival rate when exposed to M-CNH and free drug (methanol soluble at less than 0.1% concentration) (figure 5(B)), compared to A2780 cancer cells (figure 5(A)). M-CNH-treated cells were hazardous at higher and lower concentrations after 24 and 48 h. In other words, the higher the concentration, the faster the cell death occurs. When cells are treated for 24 h with either free drug or M-CNH, the concentrations of 25 and 30 μg ml−1 are shown after the cytotoxicity of both treatments has been decreased shown by the cytotoxicity test findings. As a result, agglomerates of drug molecules might develop in the environment owing to the high concentration of the drug and the enhanced interaction of molecules with each other. In other research, MARI is more effective in preventing malignant cells from growing and increasing than normal cells.
Figure 5. Measurement (A) A2780 and (B) HFFF2 viability of cells in the presence of M-CNH and free MARI in different concentrations at 24 and 48 h. Data were represented as mean ± SD (n = 3) as a percentage relative to the control.
Download figure:
Standard image High-resolution image3.5. Cell morphological analysis (AO/EB staining and DAPI staining)
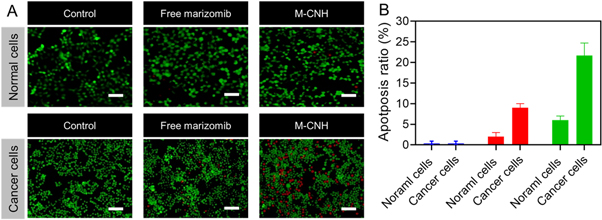
According to the AO/EB analysis, the proposed nanodrug has no cytotoxicity in normal cells, according to the AO/EB analysis. In contrast, M-CNH had higher toxicity in cancer cells than normal cells [60–62]. This study showed no substantial cell death at normal cells treated in any dose, and most cells remained alive (green). On the other hand, M-CNH-treated A2780 cells revealed both early and late apoptotic cells, stained yellow-green and red, respectively. M-CNH also reduced the number of viable cells in the treated group significantly. The treated group showed early apoptotic cells with bright green nuclei, nuclear migration, and chromatin coagulation, as well as signs of nuclear condensation apoptotic body formation (figure 6(B)). However, M-CNH groups had higher levels of early apoptotic (yellow-green) and late apoptotic (red) cells than those that used free MARI (figure 6(B)).
Figure 6. Analysis of acridine orange/ethidium bromide (AO/EB) to assess apoptosis for free MARI and M-CNH using normal cells (NIH-3T3) and cancer cells (A2780) (13 μg ml−1 concentration) by fluorescence microscopy after incubation for 24 h. Scale bar of the picture 50 μm.
Download figure:
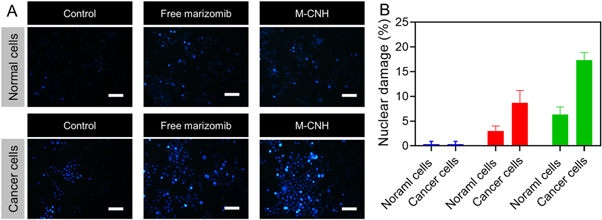
Standard image High-resolution imageAccording to the results of DAPI staining, normal cells treated for 24 h with free MARI or M-CNH (13 μg ml−1) did not affect the round nucleus, which was stained uniformly and had a clean border (figure 7(A)). More apoptosis was displayed in cancer cells treated with M-CNH rather than free MARI, and DNA fragmentation and constricted chromatin were more common in the M-CNH group (figure 7(B)).
Figure 7. Fluorescence microscopy images of normal cells (NIH-3T3) and cancer cells (A2780) incubated with free MARI and M-CNH (13 μg ml−1) for 24 h. The cell nuclei were stained with DAPI. Scale bar of the picture 50 μm.
Download figure:
Standard image High-resolution image3.6. Cellular uptake and internalization of M-CNH
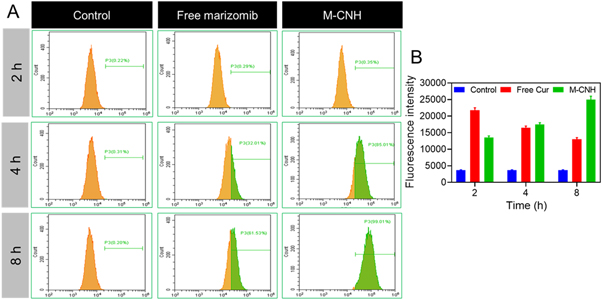
Flow cytometric analysis was performed to analyze the uptake and penetration of marizomib into A2780 cancer cells. A considerable fraction of M-CNH and free MARI entered the cytoplasm of A2780 cancer cells after 2 h of treatment with nano-drugs and free MARI, but cells untreated or treated with nanocarriers without the drug in the presence of fluorescence intensity emitted no emissions. It is clear from the quantitative fluorescence emission data acquired by flow cytometric analysis that Free drugs can freely permeate cancer cells through passive diffusion after 2 h of therapy in cells (figure 8(A)). However, the fluorescence intensity steadily declines with time. The fluorescence intensity of the M-CNH nano-drug after 8 h following cell treatment was substantially greater than 2 h compared to free drug penetration into cancer cells. M-CNH nanodrug's penetration rate has risen by around 99.02 ± 3.95% after 8 h of cell therapy, compared to the free drug (figure 8(B)).
Figure 8. (A) Flow cytometry investigation of cancer cells (A2780) incubated with free MARI and M-CNH for 24 h. (B) Quantitative analysis of mean fluorescence intensity of free MARI after incubated cancer cells with free MARI and M-CNH for 2, 4, and 8 h. Data were represented as mean ± SD (n = 3) and blank cells as the control.
Download figure:
Standard image High-resolution imageThe data may conclude that small molecules of free MARI can quickly infiltrate the cell membrane and nucleus through passive diffusion. Endocytosis is how nano-drugs penetrate cancer cells more slowly and over longer.
4. Conclusion
HA nanoparticles, unique properties such as high chemical and thermal consistency, biocompatibility, homogeneous and regular porosities, very large specific surface area with high porosity capacity, easily controlled morphological characteristics, changeable surface capacity, simple formulation and ease of transport, and concession of different molecules such as genetic makeup, proteins, antivirals, and anticancer drugs into their cavities, have been initiated as a suitable choice in medical applications. Because it is a non-intelligent material, HA alone cannot provide regulated drug delivery under a person's physiological demands. Samples in this investigation were homogeneous in structure and particle size distribution by data analysis (PSD). Nano-drug release of marizomib is also shown to be gradual and slow, with a reasonably high degree of stability. While this may seem counterintuitive at first, it may be claimed that incorporating an internal stimulus-sensitive biofilm into drug nanocarriers might increase their ability to preserve receptor molecules, thereby making it easier to regulate and enhance their characteristics. Furthermore, pharmacogenomic studies on animal models should be conducted to compare the bioavailability and effectiveness of nano-drug and free drugs in vivo. It is also important to ensure that certain cells, tissues can recognize the manufactured drug nanocarrier or organs in the body. Those specific active agents can bind to receptors on the target cell membrane.
Acknowledgments
This study was supported by Wenling Social Development Science and Technology Project (NO.2022S00139).
Data availability statement
No new data were created or analysed in this study.
Conflicts of interest
The authors declare no potential conflicts of interest.