Abstract
In the present investigation, Zero valent iron (ZVI) was synthesized using ferrous sulfate and borohydrate in the presence of EDTA and characterized by x-ray Diffraction (XRD), Energy-dispersive x-ray (EDX) and Scanning electron microscope (SEM) techniques. The prepared ZVI catalytic activity was evaluated by degrading Acid Red 1 (AR1) and Acid Green 25 (AG25) dyes. The process variables such as pH, initial dye concentration, ZVI dose, contact time, hydrogen peroxide (H2O2) and temperature were optimized for maximum dye degradation. AG25 removal was 98% at pH 4, ZVI dose 0.2 g l−1, initial dye concentration 50 mg l−1, 90 min reaction time and 8 mM H2O2 concentration, whereas pH 2, ZVI dose 0.1 g l−1, 50 mg l−1 initial dye concentration, 8 mM H2O2 and 90 min were found to be optimum for AR1 maximum degradation of 91.60%. Behnajady–Modirshahla–Ghanbery kinetic model and thermodynamic study revealed the spontaneity and endothermic nature of the process. Results revealed that ZVI has potential to degrade the dyes and could possibly be used for the degradation of dyes in wastewater.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
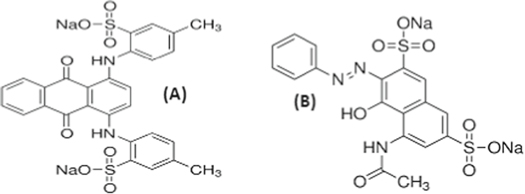
The use of synthetic dyes is increasing in dying, cosmetic, color photography parallel to the population growth. During production of dyes and dying processing, a significant amount of dyes and dyes intermediates are lost and discharged in the effluents, which are toxic and produce carcinogenic and mutagenic by-products in aqueous environment [1–6]. Dyes are the most important source of aquatic pollution. The removal of dyes from effluent is a difficult task due to their stable nature, which needs to be treated wastewater before being discharged in to water bodies. Different techniques are in practice to achieve the goal i.e., photocatalysis using nanoparticles and adsorption using adsorbents [7–10]. Acidic dyes are important class of versatile and chemically stable organic dyes and widely used for staining of wool and nylon [11]. Synthetic dyes having structural diversity, unique color and vary in functional group; usually in the form of azo, anthraquinone and triphenylemethane dyes are very common in textile application. Among dyes, azo dyes are extensively used in textile dying. The AR1 and AG25 (figure 1) have characteristic wavelength in the visible spectrum and AG25 is used for the dying of wool, silk and synthetic polyamide. It is also widely applied in leather and paper industries as well as a constituent of hair dye [12, 13]. AR1 is an aryl azo nephthol compound, extensively used for staining of wool and nylon and is non-biodegradable in nature, which needs to be treated using efficient methods to avoid negative impact on the environment and living organisms [11].
Figure 1. Structures of Acid Green 25 (A) and Acid Red 1 (B).
Download figure:
Standard image High-resolution imageRecently, nZVI has gained much importance as environmental catalyst for the treatment of wastewater versus other physical, biological and chemical methods [6, 14–21]. ZVI offers various advantages over other catalysts i.e., non-toxic, environmentally friendly, cost effective, easy to handle, higher surface area and enhanced reactivity [22]. The dye removal using ZVI happened through reductive (equations (1), (2)), oxidative (equations (3), (4)) and and coagulation/sedimentation mechanisms (equations (5)–(7)) [23]. For reduction process, e− and H2 are produced and reduced the targeted species. For oxidative degradation of dye, ·OH radicals are required and under oxic conditions, Fe generates H2O2, which resultantly gererates ·OH radicals, which destruct the organic pollutant oxidative in aqeous media [24–27]. The coagulation took place through iron oxides/hydroxides. Eluted Fe2+ is oxidized to Fe3+ and then, Fe2+ and Fe3+ react with OH− to produce iron hydroxides, which remove pollutants by coagulation/sedimentation process [28].
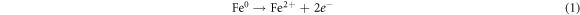
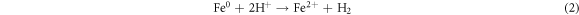
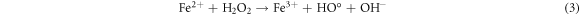
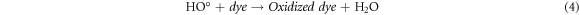
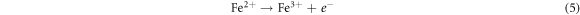
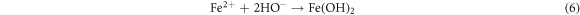
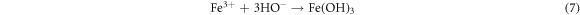
In view of efficient catalytic properties of ZVI, present study was designed to synthesize ZVI and its application for the degradation of AG25 and AR1 dyes (figure 1). Various process variables were optimized along with kinetic and thermodynamic studies. Finally, textile wastewater was treated at optimum conditions of process variables and efficiency was evaluated on the basis of degradation and improvement in water quality assurance parameters (TOC, COD) and toxicity reduction.
2. Material and methods
2.1. Chemical, reagents and instrumentation
Analytical grade regents i.e., Acid Green 25 (≥60%), Acid Red 1 (60%), FeSO4.7H2O (≥99%), EDTA (≥98.5%), NaBH4 (∼98%), HCl (37%) and NaOH (≥97.0%) were purchased from Sigma-Aldrich. Ultra-pure water was used for preparation of solutions. AFD 200, Korea analytical balance, PA 250/25.H shaker, Eyela vacuum oven, VOC-300SD oven, HI-8014 HANNA pH meter, spectrophotometer (CE Cecil 7200, UK), mechanical stirrer (Heidolph), EDX (JSM5910, INCA200/Oxford instruments, U.K), SEM (JSM-5910, JEOL) and XRD (JDX-3532, JEOL, Japan) were used throughout the study.
2.2. Synthesis procedure
In a typical procedure, 0.1 M FeSO4 in 150 ml water (4.1703 g) and 0.05 M EDTA in 100 ml water (3.7224 g) were mixed and 0.75 M NaBH4 in 100 ml water (2.837 g) was added drop wise. On slow stirring, the solution turned black. The precipitates were recovered and washed using ultra-pure water followed by absolute ethanol washing and finally, dried and pulverized [29]. The synthesized ZVI were characterized by XRD, SEM and EDX techniques [30]. Powder XRD analysis, an internal standard (α-Al2O3) was mixed with the powder for a correct calibration of the 2h-scale. Scanning electron micrographs were recorded using JSM-5910, JEOL.
2.3. Decolorization studies
Fresh stock solutions (1 M) of AG25 and AR1 were prepared in water and diluted to desired concentration. In a typical treatment procedure, the amount catalyst was dissolved in dye solution of particular concentration in 250 ml flask and stirred at 150 rpm for different time intervals. The effect of different process variables i.e., pH (2, 3, 4, 5, 6, 7 and 8), contact time (0, 15, 30, 60, 90, 120 min), H2O2 concentration (2–12 mM) Fe0 dose (0.1, 0.5, 1.0, 1.5, 2.0 g l−1), initial dye concentration (50, 75, 100, 125, 150 and 200 mg l−1) and temperature (35, 40, 45, 50, 60 °C) were investigated. The λmax of both dyes were recorded by scanning solution in 190 to 900 nm range. The residual concentrations of dyes (AG25 and AR1) were monitored at 642 nm and 506 nm, respectively and percentage color removal was estimated using relation shown in equation (8).
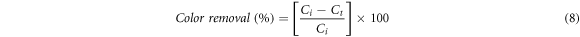
Were, Ci and Ct are the dyes initial concentrations and concentrations at time 't', respectively.
The TOC, COD and cytotoxicity of treated and un-treated wastewater were studied as precisely reported previously [31–34].
2.4. Kinetic modeling
The kinetic models (first, second order and Behnajady-Modirshahla-Ghanbery (BMG) were fitted to the experimental data as shown in equations (9)–(11), respectively. Where, Ct = concentration of dye at time 't', C0 = initial concentration, t = time, k1 = rate constant, k2 = rate constant and m and b are BMG model constants.
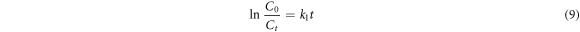
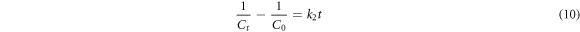
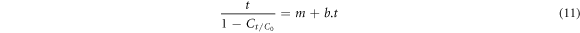
2.5. Statistical analysis
All experiments were performed in triplicate and data was averaged and reported as mean ±SD. The correlation coefficient R2 values of kinetic models were determined by statistical functions of Microsoft Excel, 2007 (Version Office XP, Microsoft Corporation, USA).
3. Results and discussion
3.1. Characterization
The synthesized ZVI phase was analyzed by XRD analysis. The ZVI showed Fe in its zero valent state and no other products were observed. The peak at 2θ value of 44.7° corresponds to Fe0 and matches precisely with JCPDS card (00-006-0696). The composition of synthesized ZVI was confirmed by EDX analysis. The EDX (figure 2(A)) revealed the presence of Fe (80.50%) and O2 (12%), Si (1.7%) and C (5.8%). The morphology of ZVI was evaluated by SEM analysis and response is shown figure 2(B). ZVI particles were spherical in shape and uniformly distributed. The diameter of ZVI was ∼100 nm. Different methods have been employed for the synthesis of ZVI particles and tested for different applications i.e., Kabir et al [35] reported the glycerol-water mixture for the synthesis of ZVI NPs under aerobic condition and the ZVI was in single phase, flower-like pattern formed from petal-like structures and clustered at the center. Similarly, nZVI on perlite was prepared and was used for the removal of azo dye CI acid orange 7 from aqueous solution in a packed-bed reactor. In present investigation, highly active ZVI particles was prepared using precipitation methods, which is simple, cost effective and easy to perform and the ZVI was in nano size and activity was promising for dyes degradation. The mechanism for the formation of ZVI is shown in equation (12) [36]. For the color removal of AG25 and AR1 using ZVI particles different process variables such as pH, contact time, Fe0 concentration, initial dye concentration, H2O2 concentration and temperature were investigated.
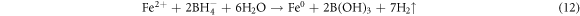
Figure 2. Energy Dispersive x-ray analysis of ZVI particles.
Download figure:
Standard image High-resolution image3.2. Effect of pH
The pH affects the solution chemistry and resultantly, the removal of dye. The effect of pH for AG25 and AR1 decolorization was studied in the range of 2–10 at 50 mg l−1 initial dye concentration, 0.2 g l−1 of Fe0 dose, temperature of 30 °C and 90 min contact time and the response thus obtained is shown in figure 3. The maximum AG25 removal was achieved at pH 4 and at this pH up to 94.77% dye color removal was achieved and then, by increasing pH to 10, the percentage removal of AG25 decreased gradually to 85%. Apparently, decolorization process was acid-driven for AG25. The color removal of AG25 is the correlated with strong reductive potential of ZVI (equations (13)–(15)). The color removal of AR1 was low versus AG25, the maximum color removal of AR1 was achieved at pH 2, that was 58.30%, and then up to pH 10 the percentage color removal decreased and reached to 41.08%. The decolorization mechanism of dye is explained in equations (14) and (15). Due to the formation of ferrous hydroxide at higher pH value, the active sites of ZVI particles were occupied by hydroxides, hence rendering the reduction capacity of ZVI [37].
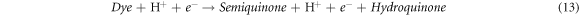
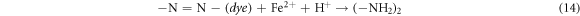
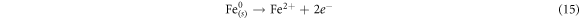
Figure 3. Effect of pH on degradation of Acid Green 25 and Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution imageIt is evident that the color removal process affected significantly with pH. Due to protonation at low pH value, the iron particles act as an electron donor, hence converting ions into atoms. Resultantly, the chromophoric group reduced and converted into amines via intermediate formation. As the solution gets more acidic, the H+ concentration increased, thus, iron particles donated more electrons and the color removal process was enhanced [38, 39]. Other than reductive process, the oxidation is also governed in the presence of Fe0. As it is mentioned in equation (16), in oxic condition, Fe0 produce H2O2, which reacts easily with the dissolved divalent iron ion (Fe2+) (equation (3)), hence strong oxidizing specie i.e., hydroxyl radicals (·OH) are produced [40, 41]. Thus, the actual color removal mechanism of rapid decolorization involves reduction and oxidation reaction. The Fe3+ and Fe2+ are inter-converted during the process [42]. Previous results are also in line with present investigation study that dyes color removal is more feasible under acidic condition in the presence of ZVI i.e., the effect of pH on AOII dye was studied, the color removal was favorable using ZVI process at pH 3.0 following first-order, while AOII dye followed the zero-order kinetics, which was correlated with ·OH radical efficiency [43]. Another comparative study of azo dyes by VZVI revealed that decolorization processes was efficient in acidic pH range and dye removal was correlated with reductive and oxidative reactions due to ZVI [37].
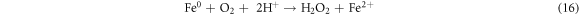
3.3. Effect of reaction time
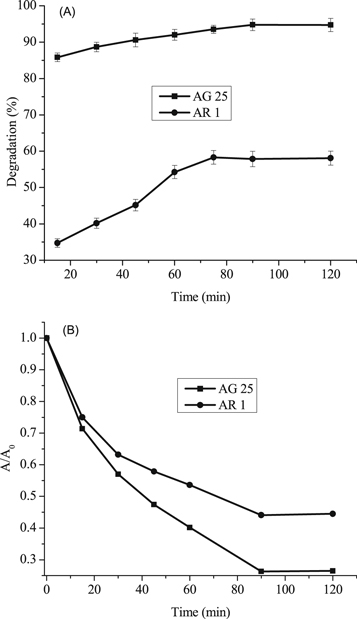
The effect reaction time was studied in the range of 0–120 min at 50 mg l−1 initial dye concentration, 0.2 g l−1 Fe0 dose, 30 °C temperature, pH 4 (AG25) and pH 2 (AR1). The removal efficiency of both dyes increased with reaction time and reached a maximum after 90 min of contact time. The maximum AG25 removal was 94.77% and then, by increasing time to 120 min, minute change was observed (figure 4(A)). The AR1 color removal was 58.308% after 90 min of reaction time and beyond this contact time, the AR1 color removal did not change significantly. Figure 4(B) (A/A0 versus reaction time) clearly indicates that the decolorization efficiency was increased with reaction time, which was rapid initially and then decreased. As it is clear from the color removal response of both dyes that the color removal rate was very fast initially and most of the AG25 degraded within first 30 min and AR1 within 60 min followed by slow color removal rate. This color removal behavior of AG25 and AR1 could be explained on the basis of easy adsorption of dye molecule [44–46] on Fe0 surface initially because of the strong adsorption and reduction ability of ZVI. At a later stage, the reduction in the color removal rate might be due to corrosion product formation, which are responsible for active sites deactivation on Fe0 surface (equations (17)–(19)) and resultantly, the color removal capacity of ZVI decreased [28]. The effect of reaction time was comparable with some previous reported studies i.e., diazepam color removal of 96% after 60 min using Fe0 ions was achieved. Under anoxic conditions, 67% color removal was achieved after 120 min [47] and acid orange II took 120 min for 90% color removal [43]. However, some other researchers reported higher color removal rate by ZVI i.e., more than 90% decolorization of methyl orange was achieved in 24 min [48] and 10 min [49]. This difference in color removal might be due to the different nature of dyes and reaction conditions [50, 51]. However, it was observed that complete dye color removal could be achieved by ZVI.
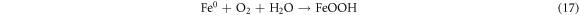
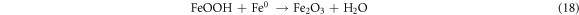
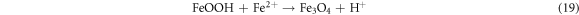
Figure 4. Effect of contact time on the degradation of Acid Green 25 and Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution image3.4. Effect of initial dye concentration
The effect of dyes initial concentration was studied in the range of 50–200 mg l−1 at pH 4 (AG25) and 2 (AR1), 0.2 g l−1 Fe0 dose, 30 °C temperature and 90 min reaction time. The AG25 color removal of 95% was observed at 25 mg l−1 concentration and by increasing the initial concentration; the AG25 color removal remained constant. The AR1 color removal was increased up to 100 mg l−1 and beyond this concentration; the AR1 removal was insignificant as a function of the initial concentration. In the case of AG25, the color removal rate remained constant beyond 25 mg l−1 and for AR1 beyond 100 mg l−1 color removal rate became constant (figure 5). Previously, the color removal of methyl orange increased from 10–20 mg l−1 initial dye concentration and beyond this concentration, the color removal was constant and the color removal at a higher initial dye concentration was enhanced using oxidants [52]. Therefore, it can be concluded that at the higher dye concentration, the color removal rate was independent to initial concentration and dyes at lower concentration might be effective for color removal under investigated conditions using ZVI.
Figure 5. Effect of dyes initial concentration on degradation of Acid Green 25 and Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution image3.5. Effect of temperature
The effect of temperature on decolorization was studied in the range 30 °C–60 °C at pH 4 (AG25), pH 2 (AR1), 0.2 g l−1 Fe0 dose, 50 mg l−1 initial dye concentration, 90 min reaction time. The reaction rate increased with temperature for AR1, whereas the color removal also increased for AG25, but slightly lower than AR1, which indicates that color removal was an endothermic process [53–55]. The color removal of AG25 was 96.1% at 30 °C, which increased to 96.8% at 40 °C and then, became constant. In case of AR1, at 30 °C, the color removal was 59.6% and reached 85.6% at 60 °C (figure 6). Previously, the decomposition of azo-dye in the presence the ZVI over the temperature range 20 °C–50 °C was reported and accelerated decolorization with temperature was observed and author correlated it with corrosion products on the surface of ZVI, which increased the surface area and at higher temperature dye color removal increased significantly [56]. Similarly, methyl orange also showed higher color removal rate at higher temperature. The color removal percentage after 20 min was 79.89% for 20 °C, 90.01% for 30 °C, and 96.75% for 40 °C and authors revealed that the dye molecules transfer from the solution phase to the ZVI particle surface at higher temperature [57]. In another study, it was observed that at low temperature, methyl orange decolorization was slow and by increasing the temperature, the decolorization process was accelerated. The decolorization efficiency within 10 min reaction increased from 72.2% to 98.3% as a function of the temperature from 20 to 40 °C. Therefore, ZVI can be used efficiently to treat dye wastewater since temperature of the wastewater is usually high when discharged from the processing units [6].
Figure 6. Effect of temperature on the degradation of Acid green 25 and Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution image3.6. Effect of Fe0 concentration
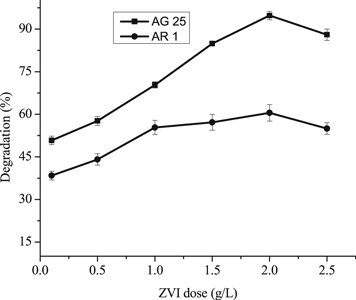
The color removal of AG25 and AR1 was studied as a function of ZVI concentration in the range of 0.01–0.25 g l−1. The AG25 and AR1 decolorization was studied at pH 4 and 2, respectively, while contact time 90 min, temperature 30 °C, dyes initial concentrations 50 mg l−1 were constant for both dyes. At 0.1 g l−1 Fe0 concentration, the color removal of AG25 and AR1 were recorded to be 50.77% and 38.38%, respectively and increased to 94.77% and 60.50% at 2.0 g l−1 of Fe0 concentration (figure 7). By increasing the Fe0 concentration, the color removal did not increase. Therefore, a concentration of ZVI of 2.0 g l−1 was optimum for maximum color removal. Previously, the effect of the ZVI dose on the color removal rate of methyl orange also showed similar trend regarding Fe0 concentration. As the ZVI dosage was increased, the concentration of methyl orange increased rapidly and the color removal efficiency was increased from about 20 to 100% [48]. The degradation of dyes (orange I, II and MO) was also promising using Fe0 and by increasing the dose, the kinetic constants for dye I and II also increased [58] and MO dye degradation was in line with present investigation as a function of Fe dose [49]. The enhanced color removal of dyes at a higher ZVI concentration might be due to availability of more surface sites for reaction at certain doses [59–62] and color removal kinetics of azo dyes increased with increasing ZVI dose [58].
Figure 7. Effect of Fe0 dose on the degradation of Acid Green 25 and Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution image3.7. Effect of H2O2 concentration
The effect of H2O2 on color removal of AG25 and AR1 was studied in the range of 1–12 mM at pH 4 (AG25) and pH 2 (AR1), whereas contact time 90 min, 30 °C temperature, 50 mg l−1 dyes initial concentration and 0.2 g l−1 Fe0 dose were constant. The color removal of AG25 and AR1 enhanced significantly by increasing the H2O2 concentration. The AG25 color removal of 98.70% at 8 mM of H2O2 concentration was achieved, whereas color removal of AR1 was 91.50% at 8 mM. Later, by increasing the H2O2 concentration up to 12 mM, the color removal remained constant (figure 8). The higher color removal rate at a certain dose of H2O2 is correlated with the generation of ·OH radical, which are strong oxidizing species and enhanced the decolorization considerably. Without H2O2, the recycling of Fe+2 and Fe+3 is a slow process, however, in the presence of H2O2, the recycling of Fe+2 and Fe+3 or its complexes dominated the production of ·OH radicals (equations (3), (20)–(27)) [63, 64]. Therefore, the catalytic activity enhanced significantly in the presence of H2O2. Moreover, at optimum condition, H2O2 is more effective and in the present investigation, 8 mM H2O2 was found to be effective and beyond this concentration, the H2O2 effect was insignificant. Previously, the same trend have been reported for the color removal of dyes in the presence of Fe0/H2O2 i.e., Red M5B, Blue MR and H-acid color removal as a function of H2O2 concentration was studied. Maximum color and COD removal were obtained in the H2O2 concentration range of 400–500 mg l−1 [65]. In another study, the decolorization rates linearly increased from 0.0232, 0.0573 to 0.0905 by incrementing the H2O2 concentrations from 5.82, 23.3 to 46.5 mM and the rate of reaction was insignificant by increasing H2O2 concentration to 69.8 mM [66] and Acid Blue 113 and acid orange 7 also showed similar color removal rate as a function of H2O2 [67, 68]. Similarly, the TOC reduction were 8, 18, 39, and 34 (%) when H2O2 was used at 100, 150, 200, and 250 mg l−1, respectively and at higher H2O2 concentration, the TOC removal efficiency decreased along with color removal efficiency. An inert oxidative film on the ZVI surface may create at higher H2O2 concentration without leaching of the Fe2+ [69] (equations (20)–(27)). At higher concentration of H2O2, it acts as an ·OH radical scavenger and hence, at a higher H2O2 concentration the color removal rate slowed down [33]. The overall mechanism of dye color removal in the presence of Fe0 and H2O2 including reduction, oxidation and coagulation/sedimentation is shown in figure 9.
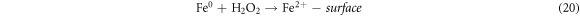
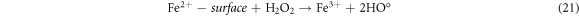
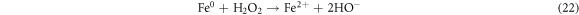
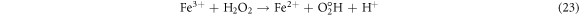
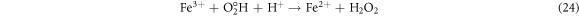
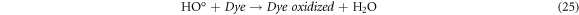
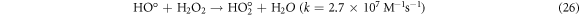
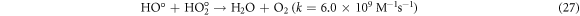
Figure 8. Effect of H2O2 concentration on the degradation of Acid Green 25 and Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution imageFigure 9. The reduction, oxidation and coagulation/sedimentation of dyes in the presence of Fe0 and H2O2.
Download figure:
Standard image High-resolution image3.8. Kinetics study
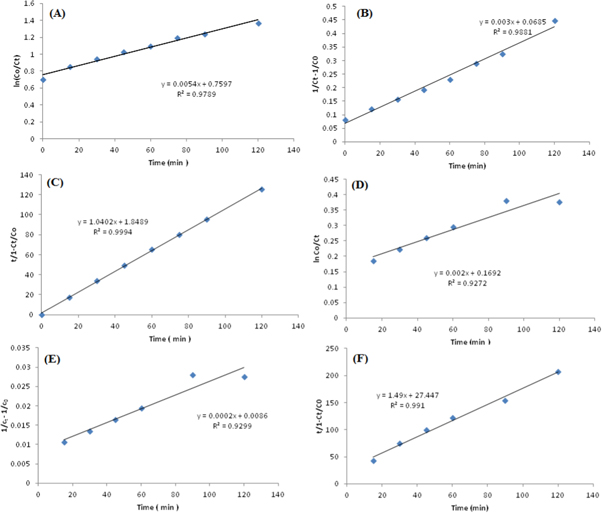
Kinetic predicts the system efficiency as a function of time and responses, thus obtained are shown in table 1. The values of correlation coefficients R2 were 0.92, 0.97 (first-order), 0.93 and 0.98 (second-order) and 0.99 and 0.99 (BMG) for AG25 and AR1, respectively, indicating that the BMG kinetic model was fitted well for the dye decolorizaton experimental data (figure 10, table 1). In BMG, b and m are two characteristic constants relating to the reaction kinetics and oxidation capacities. By plotting t/(1−Ct/Co) versus t, a straight line with an intercept of m and a slope of b was obtained. Previously, Acid Red 66 and Direct Blue 71 have also shown similar kinetic behavior [70]. In another study, the kinetics of decolorization of dyes were also studied and it had been revealed that the experimental data of dye decolorization was best explained by the BMG kinetic model [71]. Therefore, the BMG kinetic model was fitted well to the decolorization of AG25 and AR1. However, some dyes i.e., AB113, CO G, Orange II and Sunset Yellow FCF followed the pseudo-first-order kinetic when treated using ZVI [72]. Similarly, MO [49], AB24 [73], RB4 [74], triphenylmethane dye [75] also followed the pseudo first-order kinetics in the presence of ZVI. The difference might be due to the different nature of dyes and reaction conditions.
Table 1. Comparison of first-order, second-order and Behnajady-Modirshahla-Ghanbery Kinetic models for Acid Red 1 and Acid Green 25 decolorization using ZVI.
| First order Kinetic | Second order Kinetic | BMG Kinetic model | |||||
|---|---|---|---|---|---|---|---|
| Dyes | k1 (min−1) | R2 | k2 (min−1 M−1) | R2 | 1 /m | 1/b | R2 |
| Acid Red 1 | 0.002 | 0.92 | 0.0002 | 0.929 | 0.0364 | 0.671 | 0.991 |
| Acid Green 25 | 0.0054 | 0.97 | 0.003 | 0.984 | 0.54 | 0.959 | 0.999 |
Figure 10. (a) First order (b) second order (c) BMB kinetic plots of Acid Green 25, (d) First order (e) second order (f) BMB kinetic plots of Acid Red 1 using ZVI.
Download figure:
Standard image High-resolution image3.9. Thermodynamic study
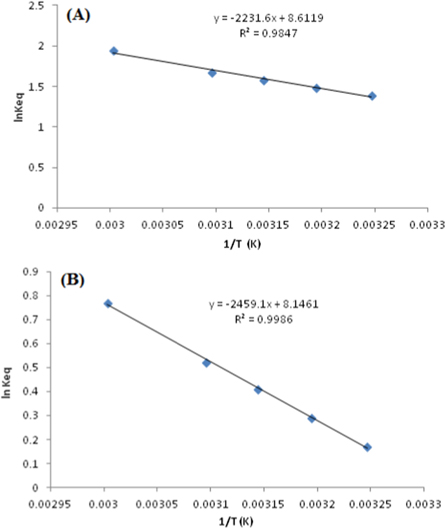
The thermodynamic [76] parameters such as enthalpy, entropy and Gibbs free energy for the decolorization of GA25 and AR1 were studied and the response thus obtained are shown in table 2 and figure 11. The ΔH° and ΔS° values for AG25 were found to be 18.548 kJ mol−1 and 71.58 J K−1, respectively, which indicates the endothermic process involved in the color removal of AG25. The entropy depicts increased the disorder of system with a temperature that was due to a breakdown of dye molecule. The positive enthalpy value clearly explains the increasing rate of reaction at elevated temperature and these finding are in line with previous studies [64]. Similar thermodynamics trend were found for the color removal of AR1. The ΔH° and ΔS° values were found to be 20.444 KJ mol−1 and 67.72 JK−1, respectively. The ΔG° is the fundamental principle for the color removal spontaneity evaluation. Negative ΔG° values showed that the AG25 and AR1 color removal process was spontaneous in nature and degree of spontaneity of the reaction increased with increasing temperature, which also revealed that reaction mechanism was energetically stable and that the rate of color removal increased with temperature [77, 78].
Table 2. Thermodynamic parameters for the decolorization of Acid Green 25 and Acid Red 1 using ZVI.
| ΔG° | ΔH° | ΔS° | ||
|---|---|---|---|---|
| Dyes | Temperature | KJ mole−1 | KJ mole−1 | J mole−1 |
| Acid Green 25 | 35 | −0.436 | 18.548 | 71.58 |
| 40 | −0.7506 | |||
| 45 | −1.079 | |||
| 50 | −1.402 | |||
| 60 | −2.133 | |||
| Acid Red 1 | 35 | −0.4569 | 20.444 | 67.72 |
| 40 | −0.6604 | |||
| 45 | −0.929 | |||
| 50 | −1.17 | |||
| 60 | −1.637 |
Figure 11. Vant Hoff thermodynamic models: (a) Acid Green 25 (b) Acid Red 1.
Download figure:
Standard image High-resolution image3.10. Treatment of textile effluents at optimized conditions
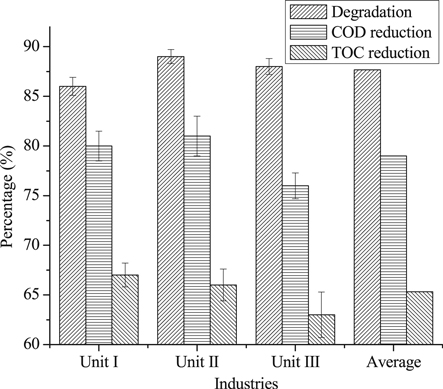
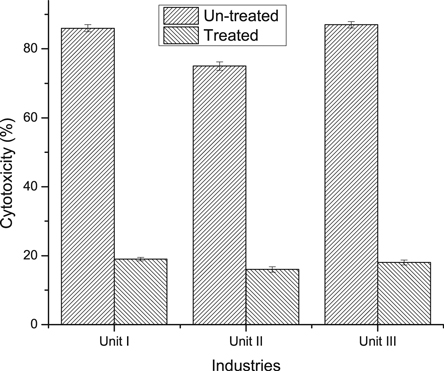
The treatment of textile effluent was done at optimum conditions for the color removal of dyes in aqueous solution i.e., pH 3, 0.2 g l−1 Fe0, 8 mM H2O2 and reaction time 90 min and 40 °C temperature. In these conditions, the color removal in the rage of 86.0%–90.0% was achieved for textile effluents. The COD as a pollution indicator which reflects the chemical quality of effluent [34], whereas TOC is suitable for determining organic matter content and provides a more accurate evaluation of the total organic compounds present in a water/wastewater, which is estimated by measuring the CO2 generated when the organic compounds are oxidized [79]. The reduction in COD was observed in the range of 76%-81%, whereas TOC reduced up to 67% (figure 12). In view of considerable color removal and COD and TOC reductions, the use of ZVI is suggested for the remediation of textile effluents. A standard test was used for cytotoxicity evaluation of un-treated and treated effluents. The cytotoxicity responses of textile wastewater before and after treatment are shown in figure 13. It was observed that the un-treated wastewater was highly toxic in nature and after treatment, the toxicity was reduced significantly. The cytotoxicity was measured by heamolytic test, which is based on RBC death in response to exposure to wastewater. Before treatment, the death rate of RBC was more than 85% and after treatment, it reduces to <20%, which revealed that up to 75% toxic agents were degraded in wastewater as a result of efficient treatment using ZVI. Hence, it was revealed that the AOP was an efficient method for the treatment of wastewater versus other wastewater methods [80–86].
Figure 12. Degradation, COD and TOC of textile effluent treated at optimized conditions of pH 3, ZVI dose 0.2 g l−1, 90 min reaction time and 8 mM H2O2 concentration.
Download figure:
Standard image High-resolution imageFigure 13. Cytotoxicity of un-treated and treated textile effluents at optimized conditions of pH 3, ZVI dose 0.2 g l−1, 90 min reaction time and 8 mM H2O2 concentration.
Download figure:
Standard image High-resolution image4. Conclusions
The ZVI were synthesized and undergone for the color removal of AR1 and AG25. AG25 color removal was achieved up to 98% at pH 4, ZVI dose 0.2 g l−1, initial dye concentration of 50 mg l−1, 90 min reaction time and 8 mM H2O2 concentration, whereas AR1 decolorization was 91.60% at pH 2, ZVI dose 0.1 g l−1, 50 mg l−1 initial dye, 8 mM H2O2 and 90 min. The dye decolorization process followed BMG kinetic model and thermodynamic study revealed the spontaneous and endothermic process for the decolorization of both dyes. Under optimum conditions; up to 89%, 81%, 67% and >75% of color, COD, TOC and cytotoxicity reductions of textile effluents were achieved. In view of promising decolorization efficiency, the ZVI application for the treatment of textile effluents is suggested and also extendable for the treatment of other toxic pollutants.
Acknowledgments
We are thankful to Dr. Hina Rizvi, Department of Environmental Sciences and Engineering, Government College University, Faisalabad, Pakistan for valuable suggestions for the revision of manuscript