Abstract
Needleless electrospinning has been a simple preparation technique for nanofiber output upscaling. However, cellulose acetate (CA) has never been tried before for its high volatility in solvent acetone. By comparing the different solvent system of acetone/dimethyl sulfoxide (DMSO), acetone/dimethylacetamide (DMAc) and acetic acid/water, the electrospun CA nanofiber was successfully prepared via metal plate needleless spinneret. The results showed that electrospun CA nanofiber could be steadily and continuously obtained in acetone/DMSO = 2/1 v/v solvent system with output around 10–20 ml h−1. The solution viscosity, conductivity and surface tension were measured with different amount of amphiphilic surfactant added. With higher amount of surfactant, the solution viscosity slightly decreased, and the conductivity increased steadily. The surface tension decreased to a stable constant with increasing surfactant amount. The correlation between the three independent variables and the average diameter and their CV value of electrospun CA nanofibers was investigated by SPSS Pearson partial correlation analysis. Solution viscosity was highly correlated with mean diameter. The linear regression analysis of the solution viscosity and the mean diameter of CA nanofibers was conducted. The predicted value of mean diameter of nanofibers had a high fitting goodness with the real value. Finally, the differences between electrospun CA nanofiber via single needle and needleless metal plate spinneret were also compared.
Export citation and abstract BibTeX RIS
1. Introduction
Cellulose acetate is a common derivative of cellulose, of which the hydroxyl groups on the anhydroglucose unit (AGU) were substituted by acetyl groups. Basically, the degree of substitution (DS) of CA is around 2.5 for better solubility in acetone for subsequent spinning process in textile industry [1]. Due to its high specific surface area, biocompatibility and high porosity, the electrospun CA nanofibers have a wide application fields like filter materials, bioremediation, tissue engineering, biosensor, drug delivery, biological separation, antimicrobial materials, etc [2].
Electrospinning has been proven to be a powerful technique for fabricating both polycrystalline and single-crystalline ultrafine nanofibril bundles [3, 4]. Along with the deepening of research, the demand for electrospun nanofiber output upscaling is in great need once it comes to products transformation. Except for the research and development (R&D) of customized biomedical materials such as tissue implants, the R&D of CA nanofiber-based products depend on not only their stable and superior properties, but the low-cost continuous preparation process. The output of single needle electrospinning is about 0.02 g h−1 [5], which is far below the demand for CA commercial products development. The typical nanofiber output upscaling methods are electrospinning via multi-needle array or needleless spinnerets. The reported output ranges from several grams to hundreds of grams per hour [6]. However, there is no published paper relate to CA needleless electrospinning since the spinnability of CA is much harder to control than polyvinyl alcohol [7, 8], polyacrylonitrile [9, 10] or polyvinyl pyrrolidone [11].
The solution properties have a great influence on CA nanofiber morphology in needleless electrospinning. Typically, the best solvent for CA is acetone. Researchers have also tried different solvent system for fiber morphology control like acetone/dimethylacetamide [12], acetic acid/water [13], dichloromethane/methanol [14], trifluoroacetic acid [15], dichloromethane/acetone [16], acetone/dimethylformamide//dichloromethane [17], methyl ethyl ketone/benzyl alcohol, acetone/dimethyl sulfoxide, acetone/benzyl alcohol, ethanol/dimethyl sulfoxide [18], etc. The beads-nanofibers structure could be obtained in acetic acid/water solvent system, and porous nanofibers could be obtained in a rapid volatilization binary solvent system of dichloromethane/acetone. However, all the solvent system presented above are applied via single needle electrospinning. As for needleless electrospinning, the solvent volatility and solution viscosity are the biggest barriers to upscaling.
In this work, CA nanofiber by needleless electrospinning with a metal plate spinneret was studied for output upscaling, which may increase the possibility of CA nanofiber related products transformation. We have selected three low toxicity solvent system for needleless electrospinning, that is acetone/DMAc, acetone/DMSO, acetic acid/water. Then, different amount of amphiphilic surfactants (SAA) was added to change solution viscosity, conductivity and surface tension. The electrospun CA nanofiber by needleless electrospinning was first conducted through acetone/DMSO solvent system. Besides, the CA nanofiber diameter and their coefficient of variation (CV) was studied by correlation analysis and simple linear regression according to solution viscosity, conductivity and surface tension.
2. Experimental
2.1. Materials
Cellulose acetate (acetyl content 39.8%) was bought from Aladdin Chemical Co. Ltd Acetone, N, N- dimethylacetamide, dimethyl sulfoxide and acetic acid were bought from Shanghai Lingfeng Chemical Co., Ltd Dodecyl trimethyl ammonium bromide (DTAB) was bought from Macklin.
2.2. Cellulose acetate needleless electrospinning via metal plate spinneret
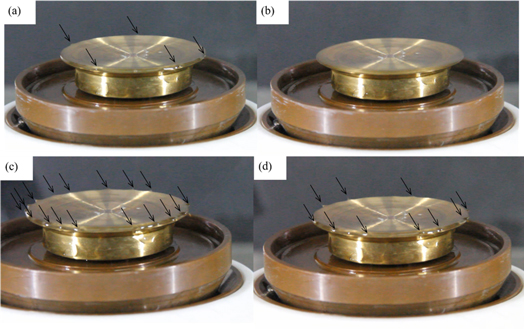
The CA powder was dissolved in different solvent system. After stirring overnight on magnetic stirrer (84-1A, Shanghai Sile Apparatus Co., Ltd), the solution was transferred to the metal plate spinneret in batches. The spinning time of each batch was 5 min, and the new spinning solution was added every 5 min. The total spinning time of each sample was 10 min. The sample was collected by a roller with melt blown nonwoven fabric. The applied voltage was 60 kV, the distance from plate to collector was 20 cm, the rotational speed of roller was 90 rpm, and the ambient temperature and humidity was 30 °C and 40%RH. The obtained nanofiber membrane was vacuum dried at 60 °C. The photo of electrospinning setup is shown in figure 1.
Figure 1. The photo of metal plate needleless electrospinning setup.
Download figure:
Standard image High-resolution image2.3. Cellulose acetate electrospinning via single needle spinneret
The CA powder was dissolved in acetone/DMSO = 2/1 v/v with a concerntion of 16 wt%. After stirring overnight on a magnetic stirrer, the solution was transferred to a 10 ml syringe with a 18G steel needle spinneret (inner diameter of 1 mm). The applied voltage was 15 kV, the distance from needle tip to collector was 15cm, the ambient temperature and humidity was 25 °C and 40%RH. The obtained nanofiber membrane was vacuum dried at 60 °C.
2.4. Characterization
The solution viscosity was measured by DV3T Rheometer (Brookfield, Germany); the conductivity was measured by Seven2Go Conductivity Meter (Mettler Toledo, Switzerland); the surface tension was measured by DCAT11 Surface Tension Meter (Data Physics, Germany); the nanofiber morphology was taken by EM 6200 Scanning Electron Microscope (KYKY, China) and the spinning status was taken by EOS7D Digital Single Lens Reflex (Canon, Japan). The fiber diameter was measured by Nano Measurer 1.2. The correlation and regression analysis were conducted on PASW Statistics 18.
3. Results and discussion
3.1. The relationship between solvent system and solution properties
The physical properties of the solvents used in this paper are given in table 1. The dielectric constants of the solvents indicate the solvent polarity, the ability of separating the opposite charges in the solution. The vapor pressure refers to the pressure of solvents in phase equilibrium. It reflects how fast the solvent evaporate into gas state. The solubility parameter of cellulose acetate is 9.5–9.7, which is close to acetone. This is also the reason why CA has better solubility in acetone than in DMSO, DMAc or acetic acid. Comparing these different solvents, other things being equal, it can be inferred that the dielectric constant and solubility parameters of acetone/DMSO lie between the other two solvent systems, while the volatility is the lowest. Only when CA was dissolved in acetone/DMSO, nanofibers could be obtained steadily and continuously.
Table 1. Solvent physical characteristics.
| Solvent | Vapor pressure (kPa, 30 °C) | Dielectric constant | Density (g/cm3) | Bolling point (°C) | Surface tension(mN/m) | Solubility parameter(J/cm3) |
|---|---|---|---|---|---|---|
| Acetone | 37.95 | 20.7 | 0.786 | 56.3 | 22.7 | 9.8 |
| DMAc | 0.39 | 37.8 | 0.937 | 166.0 | 34.0 | 11.1 |
| DMSO | 0.10 | 46.7 | 1.100 | 189.0 | 43.5 | 12.9 |
| Acetic acid | 2.74 | 6.2 | 1.050 | 117.9 | 26.6 | 12.6 |
| water | 4.24 | 80.1 | 1.000 | 100.0 | 72.8 | 23.4 |
There have been plenty of studies on the selection of solvent systems for CA electrospinning, as what have been discussed in the introduction section. The CA solution properties in different solvent systems with various concentration was shown in table 2. It can be inferred that the solution viscosity increases with the increase of solution concentration, while the conductivity remained unchanged. The viscosity of acetone/DMSO system is higher than that of acetone/DMAc system, but much smaller than that of acetic acid/water system. Acetic acid/water system has a higher conductivity, which mainly comes from water. The increase of solution conductivity increases the electrostatic force during electrospinning process, but it did not overcome the viscous force and surface tension formed by higher solution viscosity. The acetone/DMSO system reduces the volatility of solvent in solution due to the lower vapor pressure of DMSO.
Table 2. CA solution characterization.
| NO. | Solvent (v/v) | Concentration (%) | Viscosity (mPa · S) | Conductivity(μS/cm) |
|---|---|---|---|---|
| a-1 | Acetone/DMAc =2:1 | 14 | 317 | 0.253 |
| a-2 | 16 | 427 | 0.256 | |
| a-3 | 18 | 811 | 0.244 | |
| b-1 | Acetone/DMSO =2:1 | 14 | 364 | 0.238 |
| b-2 | 16 | 653 | 0.255 | |
| b-3 | 18 | 1067 | 0.230 | |
| c-1 | Acetic acid/Water = 3: 1 | 14 | 2143 | 2.839 |
| c-2 | 16 | 4537 | 2.766 | |
| c-3 | 18 | 9930 | 2.485 |
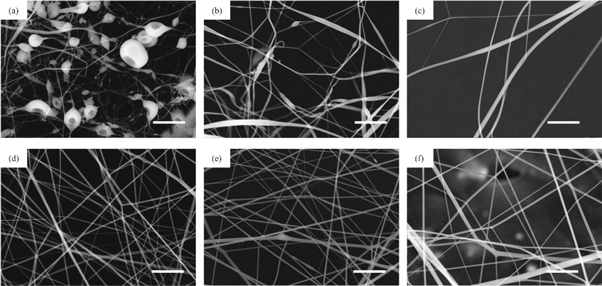
After needleless electrospinning with a metal plate, the nanofibers obtained from CA solutions in different solvents are shown in figure 2. However, the acetic acid/water system cannot get any fibers due to its high viscosity. In acetone/DMAc solvent system, when the CA concentration was 14%, there were a large number of beads in the membrane. This may due to the lack of polymer molecular intertwine in low concentration. When the concentration was 16%, there were no beads in the membrane. But the fiber diameter ranged randomly from 100 to 2000 nm. When the concentration was 18%, the solution viscosity became higher and the fibers become even thicker. The solution viscosity of acetone/DMSO solvent system was slightly higher than that of acetone/DMAc solvent system, but the volatility of the whole solvent is lower. With the increase of concentration, the average nanofiber diameter gradually increased from 273 nm, 357 nm to 760 nm. The electrospinning solution jet number decreased from 5 to 0 in acetone/DMAc solvent system in 5 min, while the solution jet number decreased from 17 to 10 in acetone/DMSO solvent system, which has been shown in figure 3. And the output was around 10–20 ml h−1.
Figure 2. Electrospun cellulose acetate nanofiber from different solvent system. (a) 14%; (b) 16%; (c) 18% cellulose acetate dissolved in acetone/dimethyl acetamide and (d) 14%;(e) 16%; (f) 18% cellulose acetate dissolved in acetone/dimethyl sulfoxide (the scale bar on the lower right corner of each picture is 10 μm).
Download figure:
Standard image High-resolution imageFigure 3. Electrospinning solution jet at (a) 0 min; (b) 5 min in acetone/dimethyl acetamide and (c) 0 min; (d) 5 min in acetone/dimethyl sulfoxide.
Download figure:
Standard image High-resolution imageThe spinnability of different solvent volume ratio of CA solution was also investigated by changing acetone/DMSO volume from 4/1, 2/1, 1/1 to 1/2 v/v. It is clearly illustrated in figure 4 that the solvent ratio had great influence on fiber morphology. When the acetone/DMSO ratio was 4/1 or 2/1 v/v, we could obtain electrospun CA fibers without beads. However, when the DMSO account greater or equal to acetone volume, plenty of solution beads accompanied by CA fiber due to the low evaporation rate of DMSO. The fibers became much thicker. Meanwhile, the solvent dissolved the fibers again when it reached the collector. Thus, the solvent ratio of 2/1 acetone/DMSO is better for metal plate needleless electrospinning considering the spinnability and evaparation rate.
Figure 4. Electrospun cellulose acetate nanofiber morphology with different acetone/dimethyl sulfoxide ratio (a) 4/1; (b) 2/1;(c) 1/1; (d) 1/2. (The scale bar on the lower right corner of each picture is 20 μm).
Download figure:
Standard image High-resolution image3.2. The surfactant influence on solution properties
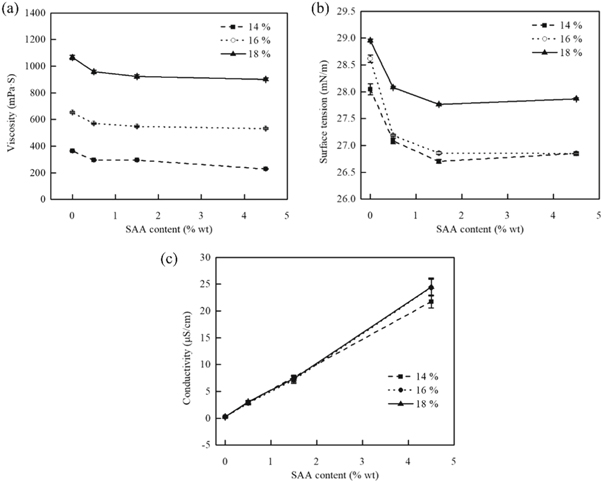
The solution properties, namely the viscosity, surface tension and conductivity, have great influence on nanofiber morphology for a specific polymer. Solution viscosity is one of the most important solution parameters affecting the morphology of fibers. Different solution concentration changes the viscosity correspondingly. Yang [19] studied the effect of different concentration and viscosity of electrospinning solution on the fiber's formation. It was found that polymer particles or beaded fibers could be formed at lower concentration due to the insufficient entanglement of molecules in solution. The surface tension of solution affects not only the formation of Taylor cone, but also the motion and splitting of jet in high voltage electric field, which ultimately influence the morphology and structure of electrospun fibers. According to Wang's study [20], the smaller the surface tension is, the smaller the critical voltage is needed for electrospinning, and the average fibers diameter is smaller. As for solution conductivity, which reflects the surface charge density of the jets, affects the axial whipping instability of the jets. With the addition of salt ions, solution conductivity become higher. The fiber diameter and beads size decreases, the diameter of the beads becomes finer and more uniform [21]. The solution characterization and their mean fiber diameter when added in different amount of amphiphilic SAA was shown in figure 5.
Figure 5. The relationship between surfactant content, cellulose acetate concentration and solution (a) viscosity; (b) surface tension; (c) conductivity.
Download figure:
Standard image High-resolution imageThe amphiphilic SAA DTAB was added in acetone/DMSO = 2:1 v/v with CA concentration of 14%, 16% and 18%, respectively. It can change the solution viscosity, surface tension and conductivity while make CA dissolve quickly in solvent. The addition of amphiphilic SAA reduces the interaction force between molecules. This is also the most possible reason for the decrease of solution viscosity after DTAB was added in figure 5(a). The viscosity increases with increasing solution concentration. As shown in figure 5(b), the surface tension of the solution decreases with the addition of a small amount of DTAB. But when the concentration exceeded 1.5%, the solution reached its critical micelle concentration, which would not reduce the surface tension of the solution anymore. The solution conductivity also increased with the increase amount of DTAB, which was owing to the presence of bromide ion and N+(CH3)3 ion in the solution. The conductivity was almost the same with different CA concentration (see figure 5(c)).
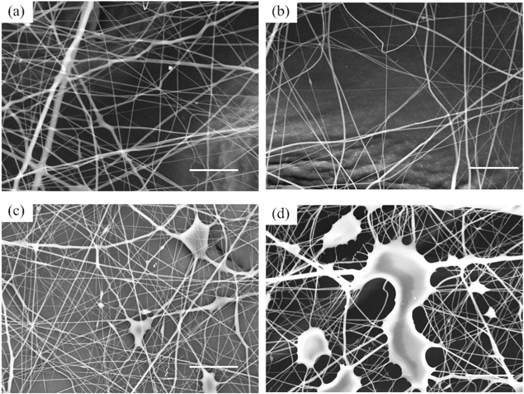
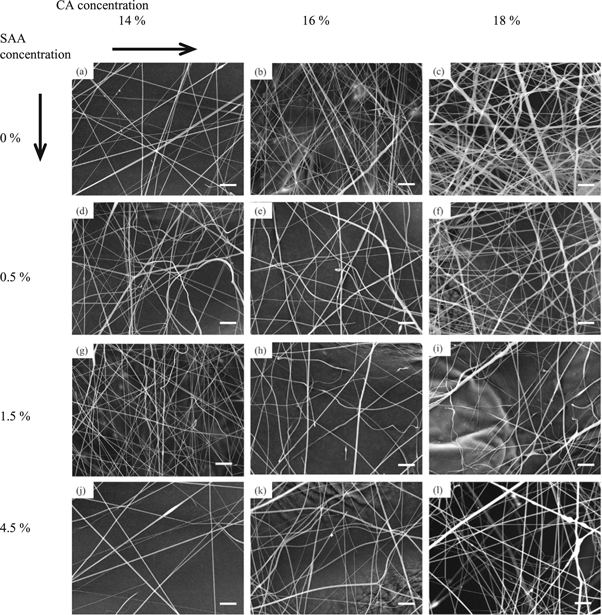
As shown in figures 6(a)–(l) together with table 3, the average diameter of the fibers decreased with the increase of DTAB content. With more ions in solution, the surface charge density of the jet is higher during the spinning process, thus making the jet thinner and intensifying the whipping process in electric field [22], resulting in finer fibers.
Figure 6. (a)–(l) The electrospun cellulose acetate nanofiber morphology with different surfactant content and solution concentration (the scale bar in the lower right corner of each picture is 10 μm).
Download figure:
Standard image High-resolution imageTable 3. The Pearson partial correction analysis of electrospun CA nanofiber mean diameter and their CV value.
| Variables | Statistics | Mean diameter | Mean Diameter CV | Viscosity | Surface Tension | Conductivity |
|---|---|---|---|---|---|---|
| Mean diameter | Correlation coefficients | 1.000 | 0.429 | 0.978 | 0.682 | −0.093 |
| Sig. (two sides) | / | 0.097 | 0.000 | 0.04 | 0.733 | |
| df | 0 | 14 | 14 | 14 | 14 | |
| Mean Diameter CV | Correlation coefficients | 0.429 | 1.000 | 0.446 | 0.629 | −0.271 |
| Sig. (two sides) | 0.097 | / | 0.084 | 0.009 | 0.309 | |
| df | 14 | 0 | 14 | 14 | 14 | |
| Viscosity, surface tension, conductivity combined | Correlation coefficients | 1.000 | −0.091 | |||
| Sig. (two sides) | / | 0.767 | ||||
| df | 0 | 11 |
3.3. The correlation and regression analysis
The addition of DTAB had different effects on solution viscosity, surface tension and conductivity. The interaction of the three factors affected both the mean fiber diameter and their CV value. According to the experimental data in table 3, the dependent variable, namely the mean diameter and their CV value, were analyzed by SPSS Pearson partial correlation to explore the three different factors on the relationship between the two dependent variables. The detailed correlation and regression analysis were further explained in supporting materials is available online at stacks.iop.org/MRX/6/1250e4/mmedia (Shown in tables 1–6).
In table 3, the solution viscosity had the highest correlation coefficients towards mean fiber diameter, while surface tension had strong correlation with mean diameter. However, all the factors had partial correlation with the mean diameter CV value, but they had less combined impact on fiber mean diameter CV value. It is a technical difficulty for electrospinning industrialization, which relate not only to the characteristic of the polymer and solvent system used, but also the spinneret shape, the ambient temperature and humidity, as well as the technical parameters control [23].
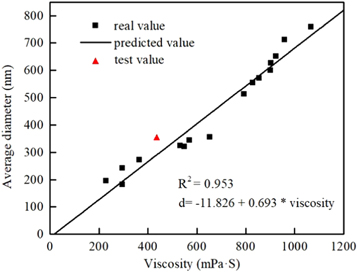
In the study we have conducted, the solution viscosity was highly correlated to the mean fiber diameter. And the solution surface tension and conductivity had no statistical significance according to the corrrelation analysis in the supporting materials. Therefore, the linear regression of mean diameter according to viscosity was conducted, neglecting the interaction of surface tension and conductivity. As shown in figure 7, the mean fiber diameter d = −11.826 + 0.693 * viscosity with a fitting goodness of 0.953. The model was tested with CA concentration of 13% (SAA 0.5%), which further testified the high correction with solution viscosity.
Figure 7. The relationship between solution viscosity and real or predicted cellulose acetate nanofiber mean diameter.
Download figure:
Standard image High-resolution image3.4. Single needle VS metal plate needleless spinneret
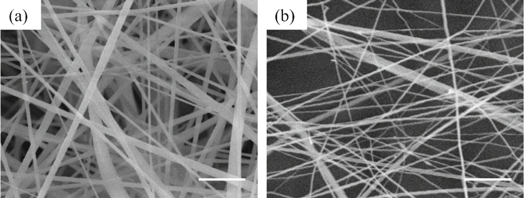
The electrospun nanofiber output of a metal plate spinneret is much higher than that of a traditional single needle spinneret. This was attributed to the simultaneous initiation of multiple charged jets at the margin of metal plate in the high voltage electric field [9]. Actually, after electrospinning for 1 h, the output for single needle and metal plate spinneret was 0.0112 g and 0.8549 g respectively. The comparasion between electrospun nanofiber morphology of single needle and metal plate spinneret was shown in figure 8. The average diameter was 414 nm and 331 nm with CV of 0.49 and 0.43 respectively. However, the nanofiber was straight with smooth surface for single needle electrospinning, while the fibers contained much twists and turns in figure 8(b). It may attribute to the interaction forces between adjacent charged jets.
Figure 8. Cellulose acetate nanofiber electrospun by (a) single needle and (b) metal plate spinneret (Take 16 wt% CA in acetone/DMSO = 2/1 v/v for example. The scale bar on the lower right corner of each picture is 4 μm).
Download figure:
Standard image High-resolution image4. Conclusions
Different solvent systems with relatively low volatility and toxicity were selected to check the spinnability of cellulose acetate solution needleless electrospinning via metal plate spinneret. The viscosity, conductivity and surface tension of the solution were measured with different amount of amphiphilic surfactant. With higher amount of surfactant, the solution viscosity slightly decreased, and the conductivity increased steadily. The surface tension decreased to a stable constant with increasing surfactant amount. The correlation between the three independent variables and the average diameter and their CV value of electrospun CA nanofibers was investigated by SPSS Pearson partial correlation analysis. Solution viscosity was highly correlated with mean diameter. Finally, the linear regression analysis of the solution viscosity and the mean diameter of CA nanofibers was conducted. The predicted value of mean diameter of nanofibers had a high fitting goodness with the real value. The differences between electrospun CA nanofiber via single needle and needleless metal plate spinneret were also compared.
Acknowledgments
This work was partly supported by the Chang Jiang Youth Scholars Program of China and grants (51773037) and the general program (51973027) from the National Natural Science Foundation of China to Prof. Xiaohong Qin as well as the 'Innovation Program of Shanghai Municipal Education Commission', 'Fundamental Research Funds for the Central Universities' and 'DHU Distinguished Young Professor Program' to her. This work has also been supported by grant (51803023, 61771123) from the National Natural Science Foundation of China to Dr Hongnan Zhang and Prof. Rongwu Wang and the Shanghai Sailing Program (18YF 1400400), the Project funded by China Postdoctoral Science Foundation (2018M640317) and the Fundamental Research Funds for the Central Universities (2232018A3-11) to Dr Zhenzhen Quan. It was also supported by the PhD Innovation Found of Donghua University (17D310123) to Mr Jiajun Wu.