Abstract
Titanium and the alloy Ti-6Al-4V are standard in implantology, despite the fact that the alloys may suffer from biomechanical incompatibility. The appropriate solution is the use of titanium β-alloys with a low modulus of elasticity and high strength. An additional advantage of these alloys is improved corrosion behaviour in environments that may contain fluoride ions, i.e. the oral cavity. Ti-25Nb-4Ta-(X)Sn alloys, where X is 4, 6, 8 and 10 weight per cent, were prepared. The phase composition, ultimate tensile strength, yield strength, Young modulus, elongation and hardness were measured. The corrosion behaviour in physiological saline, acidified physiological saline with and without the fluoride ions, was determined using, potentiodynamic polarization and electrochemical impedance spectroscopy. Bioactivity was predicted on the base of exposure in the simulated body fluid extended by impedance detection of the Ca/P layer formation. From the point of view of mechanical properties, alloys with a higher tin content are ideal for load-bearing applications. The corrosion resistance of these alloys in physiological saline is similar to titanium and significantly higher in the fluoride ions containing environment. The bioactivity test - exposure in SBF has shown quite identical results of the studied alloys and titanium, both in terms of kinetics and total composition of precipitated calcium-phosphate layer. Possible cytotoxicity effects were excluded by the exposure with murine fibroblasts. This study describes the mechanical properties and corrosion resistance in non-fluoride and fluoride containing media, predicts bioactivity and verifies non-cytotoxicity of new titanium alloys and demonstrates that they are a suitable substitute for currently the most widely used alloys (Ti-6Al-4V, Ti-6Al-7Nb) in terms of both mechanical properties and corrosion resistance for dental implants.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Titanium and the widely used alloy Ti-6Al-4V are standard in implantology due to their osseointegration ability, which is associated with corrosion resistance, sufficient fatigue strength, tensile strength and toughness [1, 2]. However, the alloys may suffer from biomechanical incompatibility. There is a difference between the elastic modulus of the material (ca 105 GPa) and the modulus of bone (16–25 GPa for cortical and 1–4 GPa for trabecular bone) [3]. This difference may cause the loss of mechanical bond because the regeneration of the bone tissue is probably controlled via mechanical stimulation of osteocytes [4–6]. This is called the stress shielding effect and is described by Wolf's law [7, 8]. Moreover, Ti-6Al-4V alloy contains the potentially harmful elements aluminium and vanadium, which can release during wear or corrosion processes [9–12].
Based on these facts, research focuses on the development of implants with an elastic module near that of bone [13]. One of the solutions to the elastic module mismatch is to use porous structures. Although these structures have better properties in the elastic area, they also have a significantly lower strength that limits load-bearing applications [14–16]. Another solution is to develop titanium alloys with a low modulus of elasticity and high strength, i.e. alloyed with β-stabilizing elements such as Ta, Nb, Zr, or Sn [17, 18]. Nevertheless, a high concentration of β-stabilizing elements results in the occurrence of a martensitic alpha phase with an orthorhombic lattice. This structure reduces tensile strength, fatigue strength and hardness [19–21]. However, the presence of alpha and omega phases can be suppressed by tin alloying, and the Young moduli of these alloys are about 40 GPa [16, 22–26]. Tin and other elements, such as Nb and Ta, also improve the corrosion resistance by enriching the passive layer by stable and non-toxic oxides such as Nb2O5, Ta2O5, and SnO2 [16, 27–30].
Corrosion in the oral cavity has its own specifics. First, there is a risk of material contact with acidic fluoride-containing media due to using prophylactic preparations to prevent caries and plaque formation [31, 32]. The combination of fluorides and low pH can dissolve the passive layer on titanium alloys and thus accelerate corrosion at inadmissible rates [32–36]. The key influence of these conditions was demonstrated both on cp-Ti, Ti-6Al-4V and Ti-6Al-7Nb alloys [37–42]. The corrosion resistance of beta alloys is generally higher in these conditions, although the effect of the combination of low pH and the presence of fluoride ions on corrosion behaviour is also not negligible. Mareci et al observed only slight improvement of corrosion resistance of Ti-Ta over the Ti-6Al-7Nb alloy in fluoride-containing electrolytes [43]. Kumar et al measured a significant increase in corrosion rate for Ti-15Mo alloy and Robin and Meirelis described the same phenomenon with Ti-23Ta alloy [44, 45]. For TMA alloy (Ti-11Mo-6Zn-4Sn), Watanabe and Watanabe showed pronounced surface changes [46]. Important problem in these environments is the change of mechanical properties associated with changes in surface state, which were measured by Walker et al on the beta III alloy (77.55% Ti, 11.5% Mo, 6% Zr, 4.5% Sn, and 0.35% Fe) after exposure in many of the prophylactic agents [47]. Loss of strength and hydrogen embrittlement was measured on a similar alloy by Kaneko et al and also noted for TMA alloy by Kwon et al [48, 49].
The aim of this work is to describe the mechanical properties and corrosion resistance in non-fluoride and fluoride media, predict bioactivity, and verify non-cytotoxicity of new complex Ti-25Nb-4Ta-(X)Sn alloys where X is 4, 6, 8 and 10 weight per cent as promising materials for use in dental implantology.
2. Materials and methods
The tested materials were titanium alloys containing 25% Nb, 4% Ta and 4, 6, 8 or 10% Sn. The alloys were prepared by six-fold arc melting and subsequent annealing at 1200 °C/4 h/vacuum (homogenization) and hot forming at 750–1050 °C. During hot forming, dynamic and post-dynamic recrystallization occurred and coarse cast grains were refined. The samples were then solution treated at 850 °C /0 .5 h/water and cold swaged with a reduction to 50%–60%.
To visualize the microstructure, the surface of the material was ground using FEPA P2400 paper, then was electrochemically polished in the mixture of concentrated nitric acid, hydrofluoric acid and sulfuric acid (volume ratio 2:2:1), in a pulsed mode at 40 volts. Thereafter, the material was then etched in Kroll's reagent.
Vickers hardness was determined on a ZWICK/Roell ZHU250 top hardness tester with a 98.1 N load (according to ISO 6507:XXXX standard). At least five values were determined for each measurement. Tensile tests have been performed using an Instron 1185 machine according to ISO 6892-1:2009 standard.
Preceding each corrosion measurement, the samples were water ground using FEPA P1200 paper, degreased by ethanol in an ultrasonic bath and sterilized by saturated water vapour at 120 °C/ 20 min in an Ecosteri sterilizer (BMT).
Physiological saline (PS) is the basic model of the human body environment, and the only aggressive components of this solution are chloride ions. This electrolyte was chosen for corrosion resistance evaluation because any other phenomena that could make the interpretation of results difficult, such as phosphate precipitation on the material surface from more sophisticated environments, were prevented. Due to the possible use of the studied alloys in dentistry, the presence acidic fluoride-containing medication was also modelled. In this study, three solutions were used: physiological saline solution containing 0.9% NaCl with an unadjusted pH, a solution with the pH adjusted to 4.2 using phthalate buffer [50] and saline with fluoride ions in form of sodium fluoride. The 200 ppm fluoride concentration was equivalent to the lowest concentration used in medical preparations. The abbreviated description of the environments following an 'environment/pH/fluoride ion concentration in ppm' pattern (e.g., PS/4.2/200) is used throughout the text. The tests were performed at 37 °C in a polymethylpentene cell. The reference electrode (silver-silver chloride electrode/3 mol L−1 KCl, henceforth referred to as SSCE) was placed in a plastic salt bridge sealed with Agar to prevent the fluoride ions from damaging the glass electrode. All potentials given in this work are related to this electrode. The counter electrode was made of glassy carbon. After a twelve hour stabilization of the open circuit potential (OCP), the electrochemical impedance spectra and the potentiodynamic curves were measured. The spectra were measured at open circuit potential with a 10 mV amplitude AC signal in a frequency range from 60 kHz to 1 mHz. The range of potentiodynamic polarization was −0.05 V/Eocp to 0.8 V/SSCE, with scan rate 1 mV s−1. The potentiostat Gamry Reference 600, controlled by Gamry Framework software, and the Gamry Echem analyst software were used for the measurement and the evaluation of all electrochemical data. Commercially pure titanium (grade 2) served as a comparative material in corrosion testing, for reasons of homogeneity and unambiguous determination of surface composition and thus a clear and exemplary corrosion response. The surface of the samples was studied using an ESCAprobe P x-ray photoelectron spectrometer (XPS) (Omicron Nanotechnology Ltd) equipped with a monochromatized Al Kα (λ = 1486.7 eV) x-ray source. The spectra were measured with a 0.05 eV energy step and were normalized to the binding energy of C1s peak (285.0 eV). After 24 h of immersion, the samples were thoroughly washed with distilled water, ethanol and acetone and transferred to the spectroscope CASA XPS. The data for the chemical state evaluation were obtained from the NIST x-ray Photoelectron Spectroscopy Database [51].
For in vitro bioactivity testing, simulated body fluid (SBF) prepared according to Mullers [52] was used. All experiments were done at the same conditions. Exposure of one sample was done in 200 ml of the electrolyte; it was not changed during exposure. The electrochemical impedance spectra were recorded every 24 h in the same range as for corrosion tests. The single frequency measurement was realised at a 2 kHz frequency with a 1 h period. Open circuit potential was also recorded during the whole exposure. Also in these tests, the comparative material was pure titanium for the same reasons mentioned above.
Cell interaction was tested with murine fibroblasts L929 (ATCC ® CCL-1™) that were cultivated in MEM (Sigma M0446) and supplemented with 10% foetal bovine serum (FBS) in the humidified atmosphere with 5% CO2 at 37 °C. Cells after thawing were passaged at least two times prior to experiments and were used maximally up to the 20th passage. Disc samples of quaternary tin alloys were sterilised at 180 °C for 2 h and used for indirect and direct (contact) tests.
Indirect tests with extracts were performed according to ISO standard 10993-5. Two samples of each type were immersed in MEM + 5% FBS and incubated at 37 °C and 130 rpm for 24 h. The surface-to-volume ratio was 82.5 cm2 ml−1. Meanwhile, L929 cells in MEM + 10% FBS were seeded into 96-well plates at a 1 × 105 cells ml−1 seeding density and incubated for 24 h. Thereafter, the medium was removed and extracts of the tested materials were added to the subconfluent layer of the L929 cell. Sterile MEM + 5% FBS medium was used as a negative (non-toxic) control. After 24 h, the cytotoxic effect of the extract was evaluated. Cells were washed with PBS and incubated with 5% WST-1 in MEM + 10% FBS without phenol red for 2.5 h. Metabolic activity was measured as the absorbance of reduced formazan at 450 nm. The measurement was done in six replicates. The cytotoxic effect was depicted as a per cent of the metabolic activity of the negative control.
L929 cells in MEM + 10% FBS were also seeded directly onto the samples placed in 12-well plates. Ti-6Al-4V alloy with the same dimensions served as a negative (non-toxic) control. Three replicates from each type were used. The seeding density was 12,000 cells cm−2 and cells were incubated for 3 days. Thereafter, samples were washed with PBS, transferred to new wells and incubated in MEM + 10% FBS without phenol red for 2.5 h. Then cells were washed onto the samples, fixed with 4% formaldehyde in PBS and the nuclei were stained with DAPI. 30. A field of view of each sample was taken at 100 × magnification using an Olympus AX 70 Provis fluorescence microscope and the nuclei were counted using ImageJ software. A statistical analysis (ANOVA followed by Tukey's test) was performed using R software.
3. Results
3.1. Material
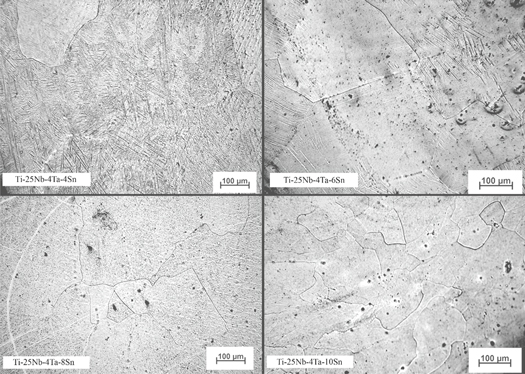
The microstructure of the studied specimens is summarised in figure 1. In the Ti-25Nb-4Ta-4Sn alloy microstructure, a needle-like phase with different directional orientation was apparent. The grain substructure was fragmented and very subtle. This alloy predominantly consisted of α' martensite. In alloys with 6% tin, grain boundaries were significantly more detectable and the default structure was beta. In the microstructure, a needle-like phase was still present but now oriented perpendicular to the grain boundaries. The grain substructure was formed by deformation strips, twins or deformation-induced martensite. Small substructure units, often located at the grain boundary, appeared in the structure—alpha precipitates. In the case of Ti-25Nb-8Ta-8Sn, the needle phase completely disappeared. A 10% tin alloy was characterized by smaller grains. Both alloys are formed dominantly by the beta phase.
Figure 1. Microstructure of Ti-25Nb-4Ta-XSn.
Download figure:
Standard image High-resolution imageFigure 2 summarizes the mechanical properties of the studied alloys. The tensile strength values of all quaternary alloys were around 800 MPa. Mean values of yield strength for martensitic alloys (4% and 6% Sn) showed considerable variance. These alloys also had higher hardness and elongation was near zero. The yield strength of β-alloy with 8% tin reached 720 ± 60 MPa and, in the case of material with 10% tin, it was 530 ± 30 MPa. Elongation of β-alloys varied around 16%. Young's modulus values were less than 70 GPa in all cases; in the case of the Ti-25Nb-4Ta-8Sn alloy, it was only 32 ± 15 GPa.
Figure 2. (a) Tensile and yield strength. (b) Young modulus and elongation. (c) hardness of Ti-25Nb-4Ta-(X)Sn.
Download figure:
Standard image High-resolution image3.2. Corrosion behaviour
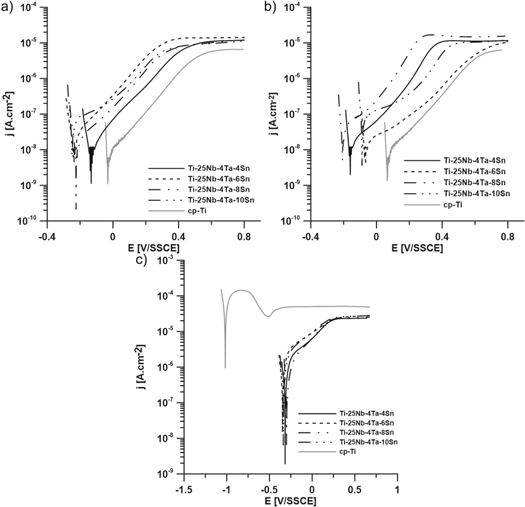
table 1 shows OCP values after 12 h of exposure. In all cases, the open circuit potential was stable and without any transients. In the presence of fluoride ions, it decreased in all cases, but in the case of the studied quaternary alloys, it did not decrease to the potential corresponding to active titanium dissolution [53].
Table 1. Open circuit potential after 12 h of exposure.
| OCP [V/SSCE] | |||
|---|---|---|---|
| Material | PS/pH unadjusted/0 | PS/pH 4.2/0 | PS/pH 4.2/200 |
| Ti-25Nb-4Ta-4Sn | −0.08 | −0.09 | −0.46 |
| Ti-25Nb-4Ta-6Sn | −0.18 | 0.01 | −0,51 |
| Ti-25Nb-4Ta-8Sn | −0.16 | −0.13 | −0.54 |
| Ti-25Nb-4Ta-10Sn | −0.18 | −0.01 | −0.44 |
| cp-Ti | −0.02 | 0.08 | −1.00 |
Figure 3 show polarization curves measured after 14 h of exposure. Anodic Tafel slopes indicate that all materials in the non-fluoride environment were in the passive state. In comparison with titanium, the corrosion potential of alloys is lower; however, the corrosion current densities remained on the same order of magnitude (10−8 A.cm−2). The change in pH resulted only in a slight change in OCP. Another situation occurred in an environment with acidic pH and fluoride ion content. The corrosion potential of pure titanium decreased to less than −1 V/SSCE, which indicates corrosion in active state. This state is also described by the shape of the potentiodynamic curve. Despite the obviously high aggressiveness of the environment, the potentials of tin alloys only dropped to around −0.35 V/SSCE. These values of corrosion potential indicate the existence of a passive layer. Trends in the curves of alloys remained the same as in fluoride-free environments, only the exchange current densities increased in order of magnitude (10−6 A.cm−2).
Figure 3. Polarization curves in physiological saline with. (a) pH unadjusted, 0 ppm F−. (b) pH 4.2, 0 ppm F−. (c) pH 4.2, 200 ppm F−.
Download figure:
Standard image High-resolution imageTable 2. Calculated values of equivalent circuits.
| R1 | CPE1 | α1 | R2 | CPE2 | α2 | Lprec | Rprec | Circuit | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Solution | Material | kΩ.cm2 | μS.s.α.cm2 | kΩ.cm2 | mS.s.α.cm2 | kH.cm2 | kΩ.cm2 | |||
| PS/unadj/0 | Ti-25Nb-4Ta-4Sn | 3500 | 41 | 0.960 | 125 | 1.1 | 0.888 | b | ||
| Ti-25Nb-4Ta-6Sn | 2440 | 48 | 0.904 | a | ||||||
| Ti-25Nb-4Ta-8Sn | 4400 | 36 | 0.924 | a | ||||||
| Ti-25Nb-4Ta-10Sn | 3700 | 65 | 0.874 | 1.6 | 1.6 | 0.927 | b | |||
| cp-Ti | 26000 | 41 | 0.937 | a | ||||||
| PS/4.2/0 | Ti-25Nb-4Ta-4Sn | 7800 | 38 | 0.925 | 0.04 | 5.1 | 0.862 | b | ||
| Ti-25Nb-4Ta-6Sn | 22000 | 32 | 0.919 | 0.50 | 3.3 | 0.751 | b | |||
| Ti-25Nb-4Ta-8Sn | 4500 | 71 | 0.902 | a | ||||||
| Ti-25Nb-4Ta-10Sn | 7800 | 82 | 0.871 | 0.89 | 2.8 | 0.893 | b | |||
| cp-Ti | 21000 | 43 | 0.929 | a | ||||||
| PS/4.2/200 | Ti-25Nb-4Ta-4Sn | 15 | 32 x103 | 1 | 2.68 | 5.1 | 0.875 | 3.42 | 0.71 | c |
| Ti-25Nb-4Ta-6Sn | 12 | 34 x103 | 0.989 | 3.41 | 1.4 | 0.932 | 5.52 | 0.66 | c | |
| Ti-25Nb-4Ta-8Sn | 14 | 30 x103 | 1 | 2.38 | 2.8 | 0.911 | 1.92 | 0.45 | c | |
| Ti-25Nb-4Ta-10Sn | 12 | 32 x103 | 0.991 | 3.26 | 3.2 | 0.914 | 5.52 | 0.64 | c | |
| cp-Ti | 0.2 | 19 x103 | 0.863 | 0.22 | 2.4 | 0.873 | b |
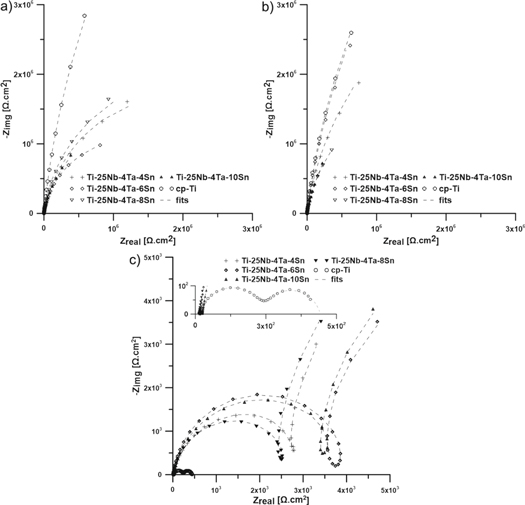
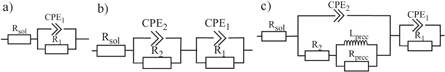
The results of impedance spectroscopy measurements are summarized in figure 4 and table 2. The equivalent circuits used for analysis are presented in figure 5. The common part of equivalent circuits consists of a resistor Rsol describing the resistivity of the solution. The constant phase element (CPE) generally replaces the capacitor in all circuits used; this replacement reflects the non-ideal behaviour of the system. This element is defined as Z = [C(jω)α]−1, where alpha takes values from 0 to 1. Values close to 1 correspond to the behaviour of the capacitor and value close to 0 to the resistor [54]. Parallel arranged R1-CPE1, in all cases except cp-Ti in PS/4.2/200, describe the properties of a compact passive layer. A circuit composed of only these elements was used to model the spectrum measured on pure titanium, Ti-25Nb-4Ta-6Sn and Ti-25Nb-4Ta-8Sn alloys in PS/unadj/0 and Ti-25Nb-4Ta-8Sn alloy in PS/4.2/0. The second circuit (figure 5(b)) is extended by another parallel R-CPE, which is connected in series to the original circuit. This pair, labelled with a lower index 2, represents the superstructure outer oxide layer on the passive layer. This extended scheme modelled other alloy spectra in non-fluoride environments. The same circuit was also used to describe the behaviour of cp-Ti in PS /4.2/200, but the interpretation is quite different here. R1-CPE1, in this case, describes the capacitive behaviour of the electric double layer and R2-CPE2 corresponds to the capacitive character of the corrosion products deposited on the surface of the material. In fluoride environments, the studied alloys also behave quite differently. An R1-CPE1 pair corresponding to the passive layer persists and there is an inductance at low frequencies. This phenomenon was modelled by a further series-connected serial-parallel couple of pairs that contained R2-CPE2 and Lprec-Rprec on the resistance branch. Inductive behaviour, represented by Lprec-Rprec, is the response to the precipitation of corrosion products, which is physically occurring on the outer oxide layer represented by the elements R2 and CPE2. The model and experimental curves show a high level of consistency, expressed as values of χ2 on the order 10−4 and below.
Figure 4. Impedance spectra in physiological saline with. (a) pH unadjusted, 0 ppm F−. (b) pH 4.2, 0 ppm F−. (c) pH 4.2, 200 ppm F−
Download figure:
Standard image High-resolution imageFigure 5. Equivalent circuits used for evaluating the impedance measurement of: (a), (b) all spectra in non-fluoride solutions and titanium in PS/4.2/200; c) quaternary alloys in PS/4.2/200 (CPE—constant phase element; R—resistance, L—inductance).
Download figure:
Standard image High-resolution imageTable 3 shows the alloy surface composition before and after exposure in all environments determined by XPS analysis. This measurement proved a significant ability of niobium to participate in the formation of surface oxides with a proportion higher than that of the bulk material. The amount of tantalum and tin in the oxide layer corresponds to bulk in most cases. The data from XPS are consistent with the thermodynamic data of the standard Gibbs free energy for the oxidation of metals, i.e. TiO2–888.8 kJ.mol−1; Ta2O5–1911.2 kJ.mol−1; Nb2O5 −1766 kJ.mol−1; SnO2 −515.8 kJ.mol−1[50].
Table 3. Weight ratio of metals in the oxide layer on surfaces determined by XPS.
| unexposed surface | PS/unadjusted/0 | |||||||
|---|---|---|---|---|---|---|---|---|
| Ti | Nb | Ta | Sn | Ti | Nb | Ta | Sn | |
| Ti-25Nb-4Ta-4Sn | 50.6 | 39.0 | 4.6 | 5.8 | 54.4 | 37.6 | 3.1 | 4.9 |
| Ti-25Nb-4Ta-6Sn | 52.7 | 36.5 | 4.5 | 6.2 | 50.4 | 38.8 | 3.9 | 6.9 |
| Ti-25Nb-4Ta-8Sn | 49.6 | 35.2 | 6.6 | 8.6 | 52.3 | 35.8 | 3.5 | 8.3 |
| Ti-25Nb-4Ta-10Sn | 48.4 | 36.1 | 5.4 | 10.1 | 48.2 | 37.2 | 3.3 | 11.2 |
| PS/4.2/0 | PS/4.2/200 | |||||||
| Ti | Nb | Ta | Sn | Ti | Nb | Ta | Sn | |
| Ti-25Nb-4Ta-4Sn | 52.7 | 37.8 | 5.0 | 4.4 | 23.6 | 51.9 | 18.8 | 5.8 |
| Ti-25Nb-4Ta-6Sn | 52.1 | 38.3 | 3.9 | 5.7 | 45.4 | 43.4 | 3.4 | 7.8 |
| Ti-25Nb-4Ta-8Sn | 51.7 | 38.0 | 3.9 | 6.5 | 44.8 | 43.4 | 2.4 | 9.4 |
| Ti-25Nb-4Ta-10Sn | 47.9 | 39.4 | 3.7 | 9.0 | 42.1 | 41.7 | 4.4 | 11.9 |
3.3. Exposure tests in SBF
Typical change of impedance spectra measured over a 24-h period during a 14-days exposure in simulated body fluid is shown in figure 6(a). In the graph, the vertical line shows a frequency of 2 kHz, where the change is well detectable in both the module and the phase. Recordings of continuous impedance response at this frequency are shown in figure 6(b). It is clear from this record that on all alloys and a pure titanium control sample, a new layer was formed after 3 days. For alloys with 8 and 10 per cent tin, the induction period of layer formation was even a few hours shorter. The presence of Ca-P layer was confirmed using SEM (figure 6(c)) with EDS. The achieved Ca/P ratios ranged from 1:1 to 1.1:1. The XPS measurement did not show the signal of an element from the material itself (Ti, Nb, Ta, Sn), only Ca and P was detected, which also confirms the surface coverage of the new layer. The area of XPS analysis was 1 mm2.
Figure 6. (a) Typical impedance spectra of alloys with Sn in simulated body fluid (displayed data belong to Ti-25Nb-4Ta-4Sn). (b) Time dependence of modulus (full lines) and phase shift (dashed lines) of samples in SBF. (c) Typical form of precipitated layer (the image shown belongs to the alloy Ti-25-Nb-4Ta-10Sn)
Download figure:
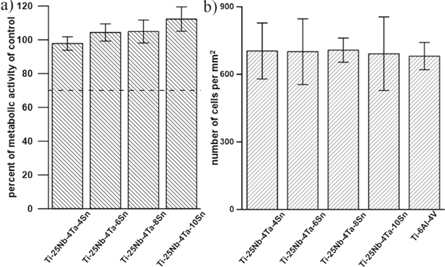
Standard image High-resolution imageFigure 7(a) shows the metabolic activity of L929 cells after 1-day of incubation with the extracts. This measurement did not show any significant difference between tin alloys and control material (Ti-6Al-4V).
Figure 7. (a) Metabolic activity of L929 cells after 1-day of incubation with the extracts of tested samples. Error bars stand for sample standard deviation from two tested replicates. (The dashed line stands for the normative limit, i.e. 70% of the metabolic activity of the control.). (b) Number of L929 cells per mm2 after 3 days of growth on materials (seeding density − 120 cells mm−2). Error bars stand for sample standard deviation from three tested replicates.
Download figure:
Standard image High-resolution imageThe three-day contact test (figure 7(b)) also did not show any statistical difference between tin alloys and control.
4. Discussion
The advantage of titanium β-alloys lies mainly in mechanical properties that are more suitable for long-times applications in bones than are those of other metallic materials used. The main objective is to achieve a similar stiffness to the bone, but without the risk of permanent deformations of the material, expressed by yield and ultimate tensile strength. The values of ultimate tensile strength for Ti-25Nb-4Ta-(X)Sn alloys (figure 2(a)) were comparable to values given by the ASTM standard for Ti-6Al-4V and are also higher than values for pure titanium given by ASTM standard [55–57]. The yield strengths (figure 2(a)) were slightly lower than the yield strength of Ti-6Al-4V alloy but were higher than Ti grade 4 [55–57]. From the point of view of biomechanical compatibility, the value of Young's module (figure 2(b)) was, in all cases, significantly closer to the cortical bone than that of Ti-6Al-4V, Ti-6Al-7Nb or all grades of titanium that achieve values higher than 100 GPa [55–58]. The positive effect of the complex composition can also be observed in comparison with the ternary Ti-30Nb-(X)Sn alloys studied by Moraes et al The addition of tantalum reduced elastic moduli in all cases: 55 GPa versus 77 GPa for 4% Sn, 45 GPa versus 49 GPa for 6% Sn, 32 GPa versus 82 GPa for 8% and 50 GPa versus 105 GPa for 10% Sn [27]. The effect of martensite in the first two studied alloys is noticeable in both elongation and hardness. The values for martensite-free β-alloys (figure 2(c)) were comparable with the usually used alloys. The hardness of martensitic alloys is comparable to titanium grade 4 and β-alloys are comparable to titanium grade 2 [55–58].
A further advantage of titanium β-alloys over commonly used materials should be improved corrosion behaviour under special conditions without the aggravation of general corrosion properties. From the polarization curves in fluoride-free electrolytes, it is obvious that the corrosion rate represented by the corrosion current density is comparable to pure titanium for alloys with lower tin contents. The material with the highest content of tin is the least resistant. Compared to cp-Ti, it has significantly more negative corrosion potential and slightly higher exchange current density. In the case of a binary Ti-Nb alloy exhibiting similar behaviour, under these conditions, the electrochemical response is given by niobium [59]. It is apparent from the surface analysis that in the oxide layer of most alloys, compared to the default state, the concentration of niobium increases during exposure.
Mechanisms of corrosion behaviour were examined by electrochemical impedance spectroscopy. In electrolyte with unadjusted pH, the spectra of all alloys and the appropriate equivalent circuits correspond to metals with a compact passive layer. R1, representing the charge transfer resistance, was on the MΩ.cm2 order of magnitude. In the case of alloys with 4 and 10 per cent tin, a second RC part must be added to model the outer oxide layer. However, compared to pure titanium, the charge transfer resistance is significantly lower, so the composite passive layer formed on the alloys in this medium had lower blocking properties. The pH decrease led to an increase in the charge transfer resistances of alloys, in the case of alloy with 6% tin, even to values identical to cp-Ti. From XPS analysis, it is obvious that this layer contains a lower concentration of tin and higher amounts of niobium and tantalum. It can be deduced that tin content in non-fluoride environments slightly reduces the effectiveness of the protective function of the passive layer. Nevertheless, the data comparison (especially the charge transfer resistance) of quaternary alloys with Ti-39Nb alloy measured under the same conditions shows the positive effect of this alloying element. The effect of alloying of tin in Ti-Nb-Sn alloys exposed in Ringer's solution was studied by Moraes et al [27]. This study also shows the positive effect of tin addition between 2 and 8% on ternary alloys, with 6% tin having the best results. In general, low tin alloying has a positive influence on the corrosion behaviour of Ti-Nb-Sn alloys in non-fluoride environments. The significantly positive influence of a small amount of tin on binary Ti-(35-X)Ta-(X)Sn alloy in physiological saline was reported by Guo et al [60]. In the case of their measurement, the corrosion current density for the alloy with 5% tin decreased by almost two orders against the unalloyed Ti-35Ta. However, Karayan et al observed a slight decrease in the corrosion resistance of Ti-10Nb-10Ta alloy in Ringer's solution compared to pure titanium [61]. The slightly negative effect of alloying by tin in a non-fluoride environment was also noticeable even in this study. Despite the results from the first two mentioned works, the slightly negative effect of alloying elements that was reported by Karayan was noticed in this study as well.
From the polarization curves measured in fluoride-containing solution, the current density increased in order in all cases, but values (as well as corrosion rate) of alloys were two orders of magnitude lower than that of pure titanium. XPS analysis shows that the passive layer is stabilized by a higher concentration of niobium and tin oxides. In the case of Ti-25Nb-5Ta-4Sn, the titanium in the passive layer was replaced by tantalum.
The different behaviour of alloys and pure titanium in fluoride environments also describe impedance spectra. While the response to cp-Ti, fitted by circuit b, corresponds to corrosion in activity, with the presence of a layer of corrosion products, the situation is different for quaternary alloys. Resistances that affects low frequencies, i.e. charge transfer resistance, are higher than an order of magnitude, so the expected corrosion rates are significantly lower. This corresponds to the polarization curves. In a previous study on Ti-39Nb alloy, this behaviour was found to be due to the presence of niobium in the alloy. However, the results of the more complex alloys with 6 and 10 per cent tin reach higher charge transfer resistance values than Ti-Nb alloys. This is, in accordance with XPS measurement, caused by the presence of stable tin oxides in the passive layer. Another positive effect could be the presence of tantalum, which, according to Mareci et al [43], could also stabilize the layer in the form of Ta2O5. This was reflected primarily in the alloy with the lowest tin content, while in other cases the stabilizing effect of tin was significantly greater.
Generally, the presence of the passive layer represented by the R1-CPE1 of the equivalent circuit (figure 5(c)) and the related relatively high charge transfer resistance table 2 in the studied alloys significantly reduces the risk of implicit failure in treatment using fluoridated medicinal products as the most effective and widely used method of treating the disease in the oral cavity. Thus, they can fulfil their function in conditions where the use of common alloys (Ti-6Al-4V, Ti-6Al-7Nb) is already dangerous.
Due to time and financial requirements of the cell-interaction and in-vivo studies, an instrumentally simpler prediction of bioactivity is needed. A relevant testing procedure based on material exposure in a model solution of inorganic blood plasma components (SBF) was formulated by Kokubo et al [62]. The criterion of the bioactivity resulting from the exposure is the formation of a hydroxyapatite layer on the surface of the biomaterial. For metallic materials, this technique has been extended with online detection of the time to start layer formation using the impedance spectroscopy [63]. This technique showed that inducing precipitation (figure 6) and the Ca-P layer composition on tin alloys is comparable to titanium. This is due to the presence of bioinert or bioactive oxides of all components on the surface of the material. The structure of these oxides probably simplifies crystallization by reducing surface free energy.
The cell-interaction testing showed that none of the tested materials was cytotoxic in the standardized indirect test with the extracts (figure 7(a)). The metabolic activity of the cells incubated with extracts was comparable to the negative control (medium with 5% FBS) and was far above the normative limit of 70%. When we used more strict conditions, i.e. incubation of a number of cells directly onto the samples, the results were also comparable with the control (figure 7(b)). These results are consistent with the very good corrosion resistance and bioactive behaviour of oxides forming a passive layer.
5. Conclusion
A series of new quaternary titanium alloys containing niobium, tantalum and a variable amount of tin was prepared in an effort to prepare a mechanically suitable, corrosion-resistant and relatively affordable alloy for dentistry. The characteristics of prepared alloys, like mechanical properties, corrosion behaviour, bioactivity and cytotoxicity, were described in this study.
From the tests results, the tensile and yield strength are sufficient for application in implantology. Young's modulus is much closer to the bone than ordinarily used alloys Ti-6Al-7Nb or Ti-6Al-4V and the danger of biomechanical incompatibility is decreased.
The corrosion resistance of these alloys in physiological saline is similar to that of commercially pure titanium. Data obtained by impedance spectroscopy revealed that all alloys in all environments were in the passive state, either as a single level non-porous barrier or a two-level layer formed by two different mixed oxides (or oxide-hydroxides).
Significantly better corrosion behaviour of alloys compared to pure titanium was found in the case of measurement in the solution containing fluoride ions. This was due to the presence of oxides of alloying elements in the passive layer of alloys, which blocked its complete dissolution and thus prevented the loss of its protective function. From the impedance spectroscopy measurement, it is evident that the character of both parts of the barrier layer has changed, with significant back precipitation of the corrosion products in the upper layer.
Testing bioactivity in simulated body fluid has also given promising results. Due to on-line impedance monitoring of the specific frequency, it was possible to accurately compare the period of the calcium-phosphate layer formation as a prediction of the rate of adherence with a tissue. Here, the results of the alloys are perfectly comparable to pure titanium, and, in the case of alloys with higher tin contents, the induction period is even a few hours shorter.
In both direct and indirect cell assays, cytotoxicity of quaternary alloys with tin was not demonstrated. Neither the measurement of the metabolic activity of L919 cells in the tin alloy extract nor the determination of the number of cells after three days in the contact test showed a negative effect compared to the control (Ti-6Al-4V) and the number of cells increased more than five times during the incubation period.
Quaternary alloys of titanium with niobium, tantalum and tin are suitable material for dental applications in terms of sufficient corrosion resistance in acid medical media containing fluoride ions, accompanied by excellent mechanical properties and reduced risk of biomechanical incompatibility. These alloys are also not cytotoxic and show comparable bioactivity to titanium in the prediction tests. For these reasons, the studied alloys appear to be a suitable substitute for commonly used Ti-6Al-4V and Ti-6Al-7Nb alloys.
Acknowledgments
Financial support from specific university research (MSMT No 21-SVV/2019).