Abstract
Surface coating of titanium (Ti) and its alloys with bioceramic materials is one of the main surface modification techniques used for developing different properties required for biomedical applications like mechanical strength, biocompatibility and corrosion behavior. In this work, pure HA and composite HA/TiO2 bioceramic coatings were prepared and deposited successfully on the surface of new biomedical Ti-Zr-Nb alloy system by sol-gel method. In order to investigate the surface properties of uncoated and coated substrates, various surface examinations were made such as surface topography, thickness, morphology and phase transformations. Furthermore, wear and corrosion properties of uncoated and coated substrates were also evaluated. The results displayed that the composite HA/TiO2 film can be considered as a potential thin film favorable for hard tissue application as it possesses an advanced crystallization and homogeneous nano-scale structure with outstanding wear and corrosion properties.
Export citation and abstract BibTeX RIS
1. Introduction
In recent years, beta (β) and metastable β Ti alloys have been extensively applied for hard tissue implant applications as they satisfy the most important properties required for biomedical applications such as high specific mechanical strength, low elastic modulus, better corrosion resistance and outstanding biocompatibility [1]. However, these alloys may be prone to a significant degradation along with releasing of various metallic ions into human body due to the weakness in their mechanical, wear and corrosion properties. Accordingly, harmfully effects on the healing of bone and the surrounding tissues are anticipated, which may guide to an extensive limitation in Ti alloys applications for implants industry [2]. Therefore, it is of a great issue to develop Ti alloys with higher performance in physiological environment using different methods and techniques. Several surface modification techniques are usually utilized as viable alternatives for developing the biological properties of Ti implants [3]. A coating technology is a leading surface modification process used for coating Ti surface with different bioactive materials using many coating techniques. The main goal of surface coating is to accomplish specific surface morphology and topography with improved surface composition and biocompatibility [4]. In this regard, sol–gel is a very common process applied for coating implants with ceramic materials as it is a simple, low-cost and essential routine to produce crystalline and high quality bioactive films at relatively low temperatures.
It is well known that this coating process is extensively applied for coating Ti surface with hydroxyapatite (HA: Ca10(PO4)6OH2) due to its sufficient bioactivity [5] as well as promoted osteointegration and mechanical stability [6]. However, HA consists mainly from Ca and P which are not existing in Ti structure. Moreover, HA possesses a brittle nature and low fracture toughness, which may normally guide to premature failure of the surface film. Therefore, serious biological problems may be occurred when Ti implants coated with HA film due to the extensive differences in chemical composition and structure between HA and Ti. This involves numerous limitations such as low mechanical properties of the film interface and poor adhesion of coating material on Ti substrate [7]. In addition, rapid resorption or degradation of HA film in biological environment may cause a severe collapse of HA film, which finally guides to an erosion of Ti implants [8]. Thus, it is of an urgent demand to find out effective solutions for these problems that are usually occurred in Ti implants coated with HA.
As an important bioceramic material, titania (TiO2) has attracted considerable attention owing to its better biocompatibility [9]. Also, TiO2 has extremely chemical affinity with both HA and Ti, highly stability and significant mechanical properties [10]. Therefore, a reinforcement of HA matrix with TiO2 was suggested to enhance the biological performance of coated Ti implants with higher chemical stability, corrosion resistance and mechanical strength [11–13]. To the best of our knowledge, study the effect of the nanocomposite coatings on the structure and the properties of biomedical β-Ti alloy is still seldom. The importance of the subject along with the increasing research interest are the driving force behind this study. In the present study, sol-gel coating technique using spray pyrolysis deposition was applied for coating new metastable β-type Ti alloy with pure HA and composite HA/TiO2 films. The structural characterizations of obtained coatings along with their effect on wear and corrosion behaviors of Ti alloy substrate were also evaluated and compared to uncoated substrate.
2. Materials and methods
2.1. Preparation of Ti alloy substrates
In this work, a metastable β Ti–15Zr–12Nb (TZN) alloy was used as a main substrate for sol-gel coating process. The TZN alloy was fabricated by a non-consumable vacuum arc melting method using the facility available at DMRL, Hyderabad, India. More details about the fabrication of the TZN alloy were mentioned elsewhere [14, 15]. Samples of size 1 × 1 × 0.3 cm from TZN alloy were cut to be used as substrates for coating process. The samples were mechanically ground with different SiC grit papers up to 1200 grit, ultrasonically cleaned with ethanol and distilled water solution for 15 min, dried and then saved in the desiccator for use. The corresponding obtained TZN alloy substrate without coating is coded as uncoated.
2.2. HA film
HA sol was principally prepared depending upon the details descripted in the literature [16]. Subsequently, HA film was produced by sol-gel method using spray pyrolysis deposition, followed by annealing treatment. For spray deposition, numerous TZN substrates were firstly heated at 100 °C to evaporate the organic materials in the precursors utilized for coating. The pressure was adjusted to be 2 bar in the compressed air line. Afterwards, the prepared solution was pumped for 10 s through particular nozzle placed 15 cm above the substrates. The coated substrates were then dried in air at room temperature for 15 min, followed by annealing treatment at 550 °C for 1 h with heating rate 5 °C min−1 in an atmospheric furnace. Ultimately, the obtained films were cooled in furnace to room temperature. The corresponding produced HA coated substrate is coded as HA.
2.3. Composite HA/TiO2 film
Composite film solution consisted of HA and TiO2 with a volume of 80 and 20 (vol%), respectively, was prepared. For TiO2 sol, nanopowder solution (30–50 nm) of 20% Ti dioxide (US Research Nanomaterials, Lnc., USA), Stock US7070, was used for the preparation of TiO2 solution. Firstly, Ti oxide (CAS♯ 13463-67-7) dispersed in water (CAS♯ 7732-18-5) was mixed for 10 min using ultrasonic mixing liquid type (MTI corporation, USA). Afterwards, both HA and TiO2 solutions were mixed and continuously stirred for 14 h, followed by ultrasonic mixing for 10 min. The HA/TiO2 composite film was produced by spray pyrolysis deposition, followed by annealing treatment. Note that the spray deposition and annealing processes of composite film were made in same conditions used for HA film. The corresponding composite HA/TiO2 coated substrate is coded as composite.
US7070, was used for the preparation of TiO2 solution. Firstly, Ti oxide (CAS♯ 13463-67-7) dispersed in water (CAS♯ 7732-18-5) was mixed for 10 min using ultrasonic mixing liquid type (MTI corporation, USA). Afterwards, both HA and TiO2 solutions were mixed and continuously stirred for 14 h, followed by ultrasonic mixing for 10 min. The HA/TiO2 composite film was produced by spray pyrolysis deposition, followed by annealing treatment. Note that the spray deposition and annealing processes of composite film were made in same conditions used for HA film. The corresponding composite HA/TiO2 coated substrate is coded as composite.
2.4. Structural characterization
Scanning electron microscope (SEM) (SEM-FEI Quanta model, Holland made) at an acceleration 12.5 KV was used to evaluate the microstructural and morphological characterization of coated substrates. Energy dispersive spectroscopy (EDS) connected with SEM device was also performed to determine the elemental composition of coated surfaces. The phase transformations in obtained coatings were analyzed using x-ray diffraction (XRD-6000, Japan). The surface roughness analysis of uncoated and coated surfaces was assessed by atomic force microscopy (AFM). A multimode scanning probe microscope (NTMDT, NTEGRA prima, Russia) was also used to obtain the corresponding images. The thickness measurements of coated substrates were determined using a digital coating thickness gauge type (LIST-MAGNETIK MEGA-CHECK Pocket).
2.5. Wear properties
The wear performance of uncoated and coated TZN alloy substrates was analyzed using dry sliding wear test. A room temperature wear test was carried out using a reciprocating pin-on-disc sliding wear tester (MICRTEST, S. A., Spain) at an applied normal force of 1 N and a sliding velocity of 200 rpm. The sliding distance and speed of wear test were chosen to be 75 m and 10 mm.s−1, respectively. The coefficient of friction, weight loss and wear rate were measured to analysis the wear resistance of uncoated and coated TZN alloy substrates depending upon the applied normal load and the recorded friction force. Also, the worn surfaces of uncoated and coated TZN alloy substrates were examined using SEM observations.
2.6. Corrosion investigation
In this work, an electrochemical workstation (Winking M Lab 200) with a standard three-electrode set up was used to investigate the electrochemical properties of uncoated and coated substrates. The test was carried out using platinum as a counter electrode, standard Ag/AgCl as a reference electrode and substrate surface as a working electrode. The electrochemical measurements were performed at 37 °C ± 0.1 using Ringer's solution as a simulated body fluid (SBF), which contains 8.60 g l−1 NaCl, 0.33 g l−1 CaCl2 and 0.3 g l−1 KCl. The corrosion investigation was carried out on a maintained exposed area of 1 cm2 using an applied potential in the range of −750 mV (SCE) to 1500 mV (SCE) with a scan rate of 1 mV s−1.
3. Results and discussion
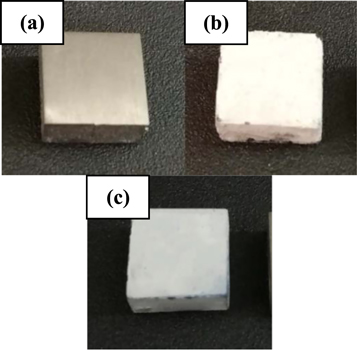
Visual assessment of uncoated and coated substrates was attained using macro scale photos, as shown in figure 1. It can be noted from figures 1(a)–(c) that both HA and composite coated substrates present an exceptional development in the coloration of their surfaces in comparison to that of uncoated surface. Furthermore, it sounds that the obtained films covered entirely the coated surfaces with optimized homogeneity.
Figure 1. Macro scale images of TZN alloy substrates (a) uncoated, (b) HA and (c) composite.
Download figure:
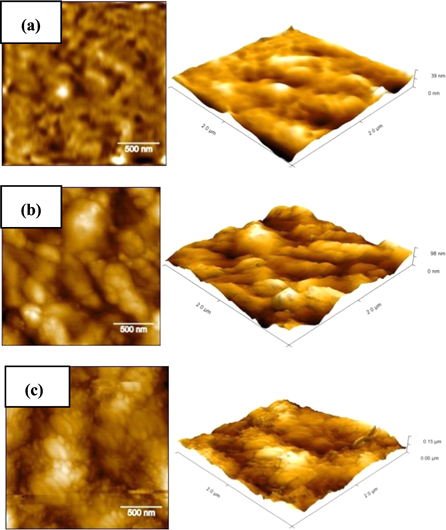
Standard image High-resolution imageThe average thickness values of obtained coatings were estimated, and the results showed that HA and composite films have micro thickness values of 22 and 41 μm, respectively. AFM images were used to analyze the average surface roughness (Ra) of uncoated, HA and composite coated substrates, as shown in figure 2. The Ra value of uncoated substrate was 9.5 nm, which presents lower Ra compared to that of coated substrates. This indicates the significant effect of different coatings produced by sol-gel process on the surface of TZN alloy. On the other hand, AFM analysis also indicated that the coated substrates are fully covered with nano-scale layers. In case of HA coating, the film is composed of nano-particles with an average grain size of about 96.8 nm, as shown in figure 2(b). The topography of this coating characterizes porous structure with an increased value of Ra (19.6 nm). This is might be due to the presence of high amount of porosity and larger agglomerations along with reduced homogeneity in HA surface structure. Moreover, the topography of composite HA/TiO2 coated substrate revealed that TiO2 is highly integrated with HA particles. It can be seen from figure 2(c) that a nano-scale structure with a higher homogeneity and smaller particle size (65.1 nm) is obtained in composite coating. The surface of composite coated substrate has reduced amount of porosity and lower surface roughness (Ra = 16.5) in comparison to HA coating. It was pointed out that the rapid attachment of the oesteoblast cells onto the implant surface can be achieved by increasing the surface roughness, which may provide a substantial improving in contact area with bone [17]. Consequently, HA and composite HA/TiO2 coated substrates induce essential developments in the bioactivity and bone bonding ability compared to uncoated substrate.
Figure 2. AFM topography images under different presentation mode and resolutions of; (a) uncoated, (b) HA and (c) composite coated substrates.
Download figure:
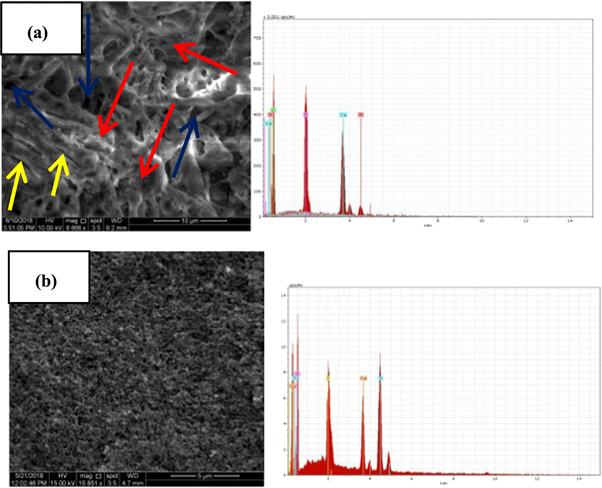
Standard image High-resolution imageFigure 3 presents the SEM images of the structure and the morphology of HA and composite coated surfaces. It can be seen from figure 3(a) that the surface is entirely covered with uniform deposition of pure HA film. The surface of HA coated substrate has irregular crystallite aggregates in plate- like shape with a thickness of 1–4 μm in various orientations (figure 3(a), red arrows). These structural aggregates can be explained as a coalescence of many nanoparticles developed on the coated surface to form coarse particles and large grains in irregular distribution. Also, some detectable micro- cracks distributed on the HA film (figure 3(a), yellow arrows) were observed. The development of cracks in deposited film is due to mutual influence of contraction and stress caused from the significant difference in thermal expansion between coating and substrate throughout thermal treatment of sol-gel process [18]. Moreover, sponge-like structure or porous structure was observed within the microstructure of pure HA film, which consists of numerous nano- and micro-pores (figure 3(a), blue arrows) in an obvious grain boundary and with dimensions of about 0.2–2 μm. It was pointed out that using implants with porous surface leads to superior attachment and proliferation of cells and bone bonding ability [19]. Also, it is important to mention here that a heterogeneous nucleation of the apatite particles can be promoted with the formation of the cracks and/or porosity in the morphology of HA coating layer. These apatite particles have a higher tendency to fill the cracks throughout their continuous growth. The corresponding EDX spectrum of HA coating (figure 3(a), right) revealed that the apatite layers were successfully synthesized on the surface of TZN alloy. It confirmed the formation of HA coating as distinct peaks related to the main elements of HA coating and TZN alloy substrate; i.e. Ca, P, O and Ti, were detected. Furthermore, the Ca/P atomic ratio of deposited HA film is 1.62, which is closer to the theoretical value of HA in human bone structure (1.67). For composite film, the coated surface has a regular thin porous structure with higher compact and homogeneity, as shown in figure 3(b). The enhanced homogeneity of coated surface is attributed to good incorporation of TiO2 with HA matrix. The deposited composite film consists mainly from nano granular-like particles with regular nano- and micro- pores. Moreover, a significant decrease in the dimensions of pores was observed in the structure of composite film compared to that of HA film. These morphological results are very identical with previous results of AFM analysis (figure 2). Moreover, it can be observed that the composite coating is highly adhered to the substrate surface without any damage, crack or disintegration, which reveals the strong sufficient strength to mechanical loads through implantation and higher bioactivity. These vital results have a good agreement with the results obtained from literature [8]. The EDS spectra (figure 3(b), right) revealed that the composite film has Ca/P ratio of 1.53. The existence of strong peaks related to Ti and O along with Ca and P peaks confirms the formation of HA and TiO2 in composite coating [20]. Furthermore, peaks other than HA and TiO2 were not seen, which confirms the purity of coating. It can be concluded that the titania phase was well embedded into HA matrix.
Figure 3. SEM photographs (left) and point EDS spectrum (right) of; (a) pure HA film and (b) composite film.
Download figure:
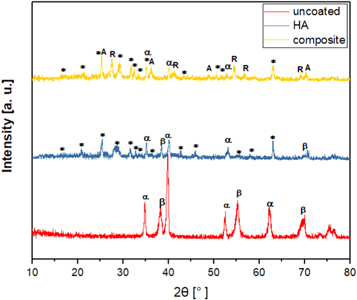
Standard image High-resolution imageThe crystal structures of uncoated, pure HA and composite films were characterized using XRD spectrum, as displayed in figure 4. The XRD analysis of uncoated substrate showed the formation of α and β phases, which are the major two phases in β-Ti alloys. For pure HA film, several major peaks at 2θ of 25.77° (002), 28.17° (102), 29.16° (210), 31.83° (211), 32.13° (112), 32.83°° (300), 34.20° (202), 39.91° (310), 46.26° (222) and 63.01° (502) attributed to HA (JCPDS pattern No. 09-0432) were detected. Furthermore, it can be noted that no sign of calcium oxide or calcium titanate in the XRD analysis of HA coating. However, there were some diffraction peaks corresponding to Ti which probably derived from TZN alloy substrate. For composite film, several typical sharp and narrow crystalline HA diffraction peaks at 25.58° (002), 28.88° (102), 28.96° (210), 31.82° (211), 32.33° (112), 32.52° (300), 34.87° (202), 49.97° (213) and 63.00° (304) were revealed. Also, some main diffraction peaks corresponding to the crystallization of TiO2 into rutile (JCPDS 00-021-1276) and anatase crystals (JCPDS00-029- 1360) were noticed. Here, it is of distinct observation that only apatite and titania phases were developed without the formation of other phases, which confirms the great thermal stability of both apatite and titania structures in composite coating derived by sol–gel process. The secondary compounds, like tricalcium phosphate, calcium titanium oxides and calcium oxide, were not observed in composite film, which may be normally seen in same film obtained by other coating techniques [21]. Moreover, it is expected that the crystallization of HA in composite film can be delayed as a result of mixing HA and TiO2 in this type of coating, which may cause a formation of weaker HA peaks [22]. It was reported that numerous biological benefits in human body environment can be obtained by introducing TiO2 in anatase form, especially improved biocompatibility [23]. It is of a great result that the formation of the rutile phase within the oxide film verifies a better stability of final oxide coating [24]. Moreover, the decomposition of HA into more soluble phases like tricalcium phosphate (α-TCP or β-TCP) may cause a significant degradation in the mechanical properties of final coating [25]. Therefore, to avoid this undesirable effect, the temperature of sintering treatment of coating was controlled to be below β-transus temperature of TZN alloy and kept at only 550 °C. The XRD patterns of composite coating also identified some diffraction peaks corresponding to Ti which might be related to TZN alloy substrate.
Figure 4. XRD patterns of uncoated, pure HA and composite coatings (*): hydroxyapatite, (A): anatase, (R): rutile, (α): α-Ti and (β): β-Ti.
Download figure:
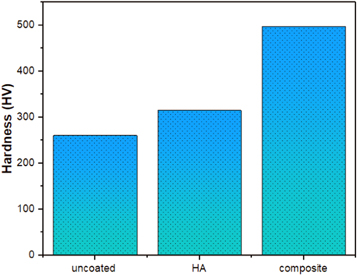
Standard image High-resolution imageAs is well identified, the biomaterials utilized for long- term implants should possess higher mechanical strength and better biological properties owing to the complex nature of the human body environment [26]. From the viewpoint of mechanical performance, the biomaterials with developed mechanical properties are appropriate alternatives for load- bearing implants. Therefore, in this study, the micro- hardness analysis, as an important mechanical investigation for the investigated coated surfaces, was performed and the results of uncoated, pure HA and composite are represented in figure 5. The uncoated substrate presented the lowest hardness value (260.6 HV) comparing with that of coated substrates, but this value is still higher than that of pure Ti (110 HV) [27]. Also, it can be observed from figure 5 that the composite coating presents the greatest hardness value (497.4 HV) compared to uncoated and pure HA. This is due to the presence of TiO2 phase in the HA matrix. It was reported that the doping of TiO2 in HA could enhance the physical reliability between the coating film and the substrate with higher inter-particle bonding, which in turn causes a considerable increase in surface hardness. Moreover, it was pointed out that the ceramic oxide films can decrease the plastic deformation of the coated substrates [28], and then increases the hardness of the coated substrates [29]. The concentration of TiO2 along with the microstructure and the morphology of coated surface are the most important parameters affected on the surface hardness. The coated surface with adherent film, high bonding strength and less porosity may offer advanced hardness values [13]. In this regard, the highest hardness of composite coating is due to its dense structure with substantial decrease in porosity of final film. Moreover, it is of an essential effect that the formation of the nanometer film in composite coating without any evident micro-cracks or detachment from the substrate (see figures 3(a) and (b)) played a key role in increasing the bonding strength with high interaction of the final film onto the substrate surface. Hence, all above results revealed that the composite film has the optimal mechanical strength when compared to HA coating.
Figure 5. Micro-hardness values of uncoated, pure HA and composite films.
Download figure:
Standard image High-resolution imageIt is well known that Ti has an ability to form a passive oxide film on its surface with thickness reaches to 1–10 nm [30]. The outstanding electrochemical properties of Ti materials under human body environment can be achieved due to the simultaneous formation of this thin surface film. However, tribological characteristics are still unsatisfactory [31]. Consequently, wear and friction of Ti implants are imperative issues as they have direct surface contact with bone tissue. Therefore, numerous biological problems, such as bone loss, inflammation and cytoxicity, could be induced due to the formation of wear debris in contact area [28]. It is very important to mention here that a coating by sol–gel is a favorable method being applied to enhance the tribological characteristics of biomedical Ti materials using an appropriate design of process parameters [32].
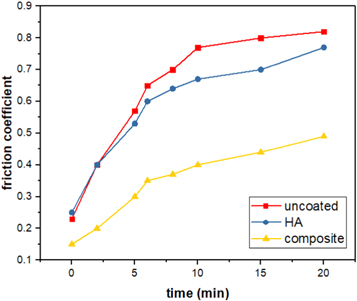
Visual inspection of uncoated, HA and composite coatings exhibited that the amount of the fractured particles on uncoated surface during wear test is higher than that on coated substrates, which may cause an increase in coefficient of friction (COF). The COF values of uncoated and coated TZN alloy substrates are shown in figure 6. It can be observed from the figure that the COF values of all tested surfaces increase with increasing test period, i.e. a continuous increase in COF with increasing the residence time of the wear test. The increase of the COF may be related to the formation of the wear debris during the friction process, which may cause an abrasive wear and final failure to surface layer [33]. Furthermore, the average COF values of uncoated, pure HA and composite coatings were 0.82, 0.72 and 0.55, respectively. This indicates that the COF values of coated substrates were significantly lower than that of uncoated substrate. In other words, the uncoated substrate has the highest value of COF which makes it more inferior compared to coated substrates. In case of HA coating, the COF value is an inferior compared to composite coated substrate, which reveals the weak influence of pure HA film for increasing the wear resistance of TZN alloy surface. This unfavorable result may be attributed to the weak interface between HA film and the surface of the TZN substrate due to the formation of internal micro-cracks onto the surface (see figure 3(a)). Consequently, micro-fracture on the interface with abrasive particles can be simply induced, which may lead to rapid failure of coating. It is worth noting that the composite coated substrate showed lower COF than HA coated substrate after completing the sliding period. This proves that the wear resistance of the composite coated substrate is the highest, which could guide to a longer wear life. This optimal finding is greatly associated with the presence of nano-scale homogenous structure of composite film with outstanding surface bonding between composite film and TZN alloy substrate (see figure 3(b)). Also, it is important to mention here that the significant reduction in COF of composite coated substrate may be resulted from the considerable increase in some vital properties of formed film especially structure stability, surface hardness, film thickness [34, 35] and roughness [36].
Figure 6. Coefficient of friction values of uncoated, HA and composite coatings.
Download figure:
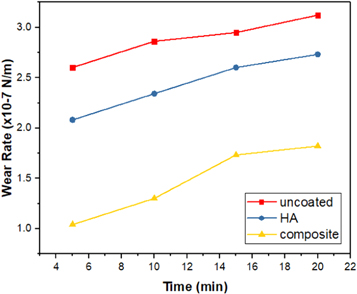
Standard image High-resolution imageSimilarly, figure 7 demonstrates the wear rates of uncoated, HA and composite coatings. It can be seen that the value of wear rate of uncoated substrate is higher compared to that of coated substrates. This is owing to lower hardness value of uncoated substrate comparing with coated substrates (see figure 5), which can induce a significant increase in wear rate. On the other hand, among coated substrates, composite coating possesses the optimum result regarding the wear rate.
Figure 7. Wear rates of uncoated, HA and composite coatings.
Download figure:
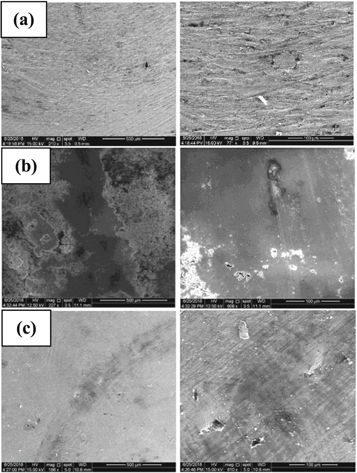
Standard image High-resolution imageUsing SEM observations, the worn surfaces of uncoated and coated substrates were evaluated in order to attain more details about the mechanisms of friction and wear. Figure 8 shows the wear tracks of uncoated, HA and composite coatings after completing the wear test. It can be seen that the track width of the uncoated substrate (figure 8(a)) is so large, which indicates that this surface suffered from severe adhesive wear and plastic deformation between the pin and the substrate during the wear test [37]. In contrast, the coated substrates (figures 8(b) and (c)) had narrow track width, which reveals that the wear resistance of TZN alloy developed after sol–gel coating process. In addition, the narrowest wear track was observed on composite coated substrate (figure 8(c)), which indicates that this film has an excellent wear resistance among the coated substrates. This might be attributed to the high surface hardness and film thickness of composite film compared to HA film. Also, the worn surface of composite coated substrate was relatively smoother than HA coated substrate. Also, a large delamination and plentiful fine wear particles were formed on the surface of HA (see figure 8(b)), indicating the possibility of occurring sudden rupture through wear process. The worn surfaces image of composite coated substrate showed that an evident deformation occurred on the surface, which discloses the optimum result of the wear resistance, as shown in figure 8(c). The plastic deformation is may be occurred due to the nano-scale structure of composite film (see figures 2(c) and 3(b)). It is well identified that the ceramic coatings in nano-scale display some plastic deformation even under room temperature [38]. The deformation is so probably responsible for prevention or decrease the potential creation of micro-fractures, which could lead to a significant decrease in friction coefficient and abrasive particles. It can be concluded that the wear mechanism of composite coated substrate is featured with deformation and abrasive wear without apparent fracture, whereas the wear mechanism of HA coated substrate is dominated by microfracture and abrasive wear.
Figure 8. SEM images of wear tracks at low magnification (left) and high magnification (right) of (a) uncoated, (b) HA and (c) composite.
Download figure:
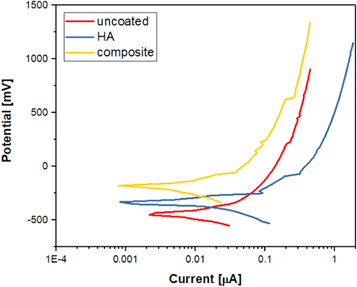
Standard image High-resolution imageElectrochemical properties of uncoated, HA and composite coatings were evaluated using a potentiodynamic anodic polarization, as shown in figure 9. The average values of the major corrosion parameters obtained from Tafel curves are summarized in table 1.
Figure 9. Polarization curves of uncoated, HA and composite coatings.
Download figure:
Standard image High-resolution imageTable 1. Potentiodynamic polarization parameters of uncoated, HA and composite coatings.
| Substrate | βa (mV) | βc (mV) | Ecorr (mV) | icorr (μA cm-2) | Rp (μΩ cm2) | CR (mpy) |
|---|---|---|---|---|---|---|
| Uncoated | 323.6 | 194.3 | −389 | 6.61 | 7.99 | 1.887 |
| HA | 84.1 | 77.6 | −340 | 2.97 | 5.91 | 0.841 |
| Composite | 110.5 | 129.6 | −212 | 1.90 | 13.64 | 0.542 |
It can be found from the analysis of corrosion potential values that they increase in the following order: uncoated substrate (−389 mV (SCE)) < pure HA film (−340 mV (SCE)) < composite film (−212 mV (SCE)). These results disclose that HA and composite coatings have more nobler values of Ecorr compared to uncoated substrate. Therefore, the coated substrates with HA and composite films have a considerable increase in corrosion resistance due to an exceptional protective nature of coated films against corrosion attacks of SBF. However, HA film presented an obvious deterioration in anticorrosion properties as its corrosion rate is higher than that of composite film. The low corrosion resistance of HA film can be related to its surface morphology which contains some cracks and more pores (see figure 3(a)). As a result, this leads to a simply penetration of SBF into surface, which in turn causes a damage in HA film along with an imperative missing of its barrier properties. In contrast, the coating with composite film increased significantly the corrosion resistance of TZN alloy substrate in Ringer's solution. The data of icorr and Rp also displayed a similar trends regarding the corrosion behaviors of coated substrates. The excellent corrosion resistance of composite film can be attributed to its uniform, dense and compact surface structure as well as an outstanding adhesion strength of this film into TZN alloy surface (see figure 3(b)). Furthermore, the thickness of the composite layer was measured to be 41 μm, which is more than that of HA film (22 μm). It is well identified that the corrosion resistance of the biomaterials can be developed with increasing the thickness of surface coatings [39]. That is to say, the composite film offers an enhanced anticorrosion properties for TZN alloy under the sever conditions of the physiological solution. Thus, this type of coating can be exploited as a promising biofilm for hard tissue implant applications.
4. Conclusion
The main conclusions of this work can be summarized as follows:
- In both coatings, HA and composite, the coated substrates are fully covered by nano-crystalline films composed of nano-particles in an average grain size of about 96.8 and 65.1 nm, respectively.
- HA film possesses irregular crystallite aggregates in plate-like shape with numerous nano- and micro-pores and some detectable micro-cracks distributed on the surface. In contrast, composite film consists mainly from nano granular-like particles and regular nano- and micro-pores in a significant decrease in their dimensions along with compact structure in higher homogeneity without any damage, crack or disintegration.
- Composite film shows a stable structure with an outstanding crystallization and higher surface mechanical bonding due to the good incorporation of TiO2 with HA matrix without any decomposition of HA into more soluble phases.
- The composite film enhances greatly the corrosion resistance of TZN alloy in SBF.
- Further studies are required to estimate the adhesion of proteins and proliferation of human osteoblasts.
Acknowledgments
The authors of this work are pleased to acknowledge DMRL, Hyderabad, India for manufacturing titanium alloy.