Abstract
Fabrication of tissue-/organ-like structures at arbitrary geometries by mimicking the properties of the complex material offers enormous interest to the research and clinical applicability in cardiovascular diseases. Patient-specific, durable, and realistic three-dimensional (3D) cardiac models for anatomic consideration have been developed for education, pro-surgery planning, and intra-surgery guidance. In cardiac tissue engineering (TE), 3D printing technology is the most convenient and efficient microfabrication method to create biomimetic cardiovascular tissue for the potential in vivo implantation. Although booming rapidly, this technology is still in its infancy. Herein, we provide an emphasis on the application of this technology in clinical practices, micro- and nanoscale fabrications by cardiac TE. Initially, we will give an overview on the fabrication methods that can be used to synthesize the arbitrary 3D components with controlled features and will subsequently highlight the current limitations and future perspective of 3D printing used for cardiovascular diseases.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Additive manufacturing (AM), often referred to as three-dimensional (3D) printing, is a highly attractive technological approach for the layer-by-layer fabrication of complex structures [1, 2]. This strategy offers abundant benefits such as ease-of-use, cost-effectiveness, reliability, and applicability to various compatible materials, which enables the utilization of 3D printing in diversified fields of science and technology [3]. More often, the translation of 3D printing to biofabrication/3D bioprinting is achieved by building the high-resolution 3D structures smearing biological materials, in particular, that mimic the growth mechanisms [4]. However, AM is different from biofabrication due to the need for physiological conditions during processing that limits the utility of other suitable AM technologies. To this end, 3D bioprinting has heralded the rapid and extensive growth in medicine by the fabrication of materials and cells to produce realistic tissue- as well as organ-like structures [2, 5–7].
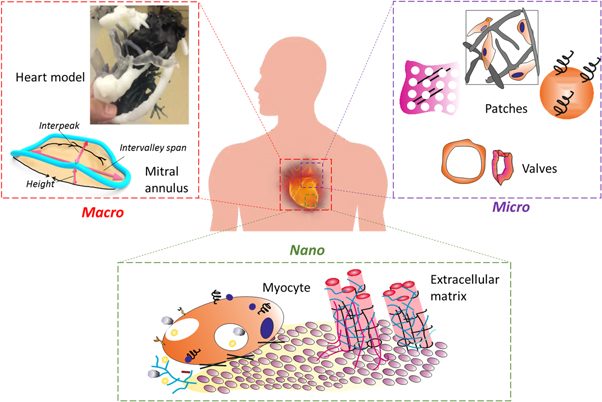
In recent years, 3D printing has garnered the enormous, fascinating interest of surgeons and researchers towards cardiac surgery-related applications, such as surgery education, pre-operational planning, intra-operational guidance and engineered cardiac tissues (ECT) for implantation [7, 8]. In this context, various 3D arbitrary components (figure 1), including the whole heart model, cardiac tissues, valves, and blood vessels have been successfully developed by different 3D printing-based tissue engineering (TE) approaches [9]. Few recent perspectives related to these concerns have been focused on cardiac-related therapeutic applications [10, 11]. However, this review emphasizes the application of 3D printing technology in manufacturing different components of the cardiovascular system at an arbitrary gauge, ranging from macro-, micro- to nanoscale, to benefit the surgery practices for various cardiac-related complications such as myocardial infarction (MI) and others (figure 2).
Figure 1. Graphical illustration representing the applicability and size of macro-, micro- and nanoscale arbitrary 3D components in cardiac tissue engineering in a contrary-wise manner (left). Illustration showing the potential applications of respective 3D-printed components (right).
Download figure:
Standard image High-resolution imageFigure 2. Schematic representation of fabricated 3D components or models at an arbitrary size ranging from macro- (heart model, mitral annulus (MA)), micro- (cardiac patches and valves), to the nanoscale (such as carbon nanotubes and others) for cardiac surgery. Heart model reproduced from [12], copyright 2016, with permission from Elsevier.
Download figure:
Standard image High-resolution imageMethods of manufacturing 3D models
3D printing is perhaps one of the most extensively used techniques so far to obtain suitable physical models at arbitrary sizes with precise geometry for diverse applications. In general, this technology works based on the transformation of computer-aided design (CAD) structures into a desired model [9, 13]. The most commonly used approaches in developing 3D-printed models include selective laser sintering (SLS), fused deposition modeling (FDM), inkjet printing (IJP), multi-jet modeling (MJM), extrusion-based approach, and laser-based stereolithography (SL) [3, 9, 13–15].
In an SLS process, a high-powered laser (such as carbon dioxide laser) is used to sinter/fuse the powdered material, i.e., smaller particles of plastic, metal, or ceramic powders into a solid complex 3D object following a definite pre-designed CAD file pattern (figure 3(a)) [13]. The significant advantage of the SLS process is that no sign of clogging is seen as this process is devoid of nozzles and can be able to print a broad range of materials [15]. The SLS process yields products with great flexibility and high geometric accuracy, but it is limited in its application because of the utilization of relatively expensive high-powered lasers.
Figure 3. Schematic illustration showing various rapid prototyping-based fabrication methods. (a) SLS process, (b) FDM, (c) IJP/MJM, (d) extrusion-based printing and (e) laser-based SL.
Download figure:
Standard image High-resolution imageFDM is another AM technique that utilizes a plastic (such as, acrylonitrile butadiene styrene (ABS) polymer) filament as a substrate that is forced through an heated extrusion nozzle, melted, and deposited directly on a water-soluble material as temporary support as guided by the CAD pattern (figure 3(b)) [3]. The FDM process yields products at an excellent geometric accuracy since it utilizes durable material such as plastic and metals and the end products are allowed to be sterilized. The only disadvantage of FDM is that the preparation of 3D models and removal of support structures are time-consuming [16].
IJP is another critical approach of AM technology that is favorable for generating arbitrary structures by depositing the small drops of materials (i.e., ink) using a jet onto a supporting substrate (figure 3(c)). More often, it is capable of printing microstructures with planar or thin 3D features. IJP is an inexpensive process with a higher-throughput compared to other AM techniques and has been used to fabricate devices for diverse applications [3]. Based on the flow of the ink, these printers were classified into two types, one of them is continuous-based, and the other is drop-on-demand-based printer [14]. The continuous-based model has high-speed capabilities and able to print the models with large-scale areas. Whereas, drop-on-demand type model is preferred to accomplish cost-effective models with high-precision [9].
Typically, the working principle of the MJM process is almost similar to a standard IJP process but happens to be favorable in a 3D space by printing a water-based adhesive in the desired shape of each cross-section as determined by the CAD file. MJM is the fastest AM method as the deposited layers are hardened rapidly, and the process is continued until the model is complete. This approach could also accommodate the printing of tinted prototypes for better visualization of various fragments in the model. Despite its advantages, MJM approach has certain drawbacks such as it produces models with less geometric accuracy, completely opaque and, not as mechanically strong as compared to the products obtained from other AM approaches [13].
The extrusion-based approach is another important and cost-effective AM method that is highly favorable to print a wide-variety of materials [9, 15, 17] such as high viscosity cell-laden hydrogels and other biomaterials [9, 15]. This approach is based on the computer-controlled deposition/extrusion of inks, which are allowed to build 3D-microstructures through a layer-by-layer building sequence without utilizing any sophisticated tools (figure 3(d)). The only minor disadvantage of this approach is that the high viscosity of materials and nanofiller clusters may clog the nozzle [18].
Currently, SL is the most widely used AM technology that builds the 3D components by solidifying the deposited layers of a photosensitive resin (figure 3(e)) [13, 19]. The computer-controlled UV-laser beam polymerizes the resin instantaneously in a defined pattern and eventually results in its solidification. The instrument setup with a movable platform facilitates the space for the instantaneous formation of the new layer, and the process is continued until the model is complete. This technique is advantageous over other AM approaches in generating 3D components with a high geometric accuracy. However, its application is limited due to the long manufacturing time, and high cost. Also, this technique creates transparent models [13].
Macroscale structures for realistic modeling
3D printing enables the creation of realistic anatomic models using the patient's imaging data set obtained from various techniques such as computer tomography (CT), ultrasound, and others [20, 21]. More often, these macro sized models (table 1) allow the direct 3D visualization of cardiac anatomy to the surgeon for pre-surgical preparation, thus facilitates the device planning and cannula placement during complex congenital lesions and others [22]. The printers are capable of producing 3D models that can vary widely depending on the print size (build volume), the material used for printing, layer resolution, and support material solubility. Additional appendages can also be printed to protect any overhanging parts from crumpling before congealing, and few have an ability to reproduce the different colored appendages within the same model, for instance, printing tumors within the myocardium [12]. At each stage of fabrication, including image acquisition, data processing, and manufacturing, cardiac surgeons need to communicate with the technologists to obtain a patient-specific realistic model for a better surgical procedure [23, 24].
Model printing for congenital heart disease (CHD) surgery
Despite the success of surgical therapy and neonatal screening, the patients with CHD at an adult age is still rapidly growing. The heterogeneity and complex anatomic characteristics of the heart make them essential for personalized and precious supervision, thus amplifying the widespread application of 3D printing in CHD management. The approach of creating patient-specific 3D-printed cardiac models (ventricular assist devices (VAD) (figure 4(a)) will certainly narrow the gap between the patients with and without CHD, who are being offered these potentially life-extending therapies (figure 4(b)) [22, 23, 25]. Moreover, the applications of 3D-printed models in pediatric patients with CHD include visualizing intracardiac spatial anatomy for repairing ventricular septal defects (VSD), a double outlet right ventricle, and tetralogy of Fallot with major aortopulmonary collateral arteries (figure 4(c)) [26]. 3D prototypes of malformed hearts of all the age groups have been prepared and used for various reasons, specifically exploring the spatial relationship for better understanding during pre-surgical training [24].
Figure 4. Various macro-size 3D models for surgery, (a) the posterior aspect of corresponding printed model reflecting a 37 year old patient's heart with tricuspid atresia D-transposed great vessels s/p Fontan procedure with persistent ascites, and atrial arrhythmias, and can be used to plan for VAD placement. Reproduced from [25], copyright 2016, with permission from Elsevier. (b) 3D-printed heart model by FDM for CHD. Reproduced from [23]. CC BY 4.0. (c) 3D model demonstrating the intracardiac anatomical features. Reproduced from [26] 2016 © Springer Science+Business Media New York 2015 With permission of Springer. (d) 3D-printed model of the aortic arch after the frozen elephant trunk (FET) procedure showing the prostheses replacing the ascending aorta (+) and the supra-aortic vessels (*), arrows indicating the stent. Reproduced from [27], copyright 2014, with permission from Elsevier. (e) 3D model used in thoracic surgery for superior sulcus (Pancoast) tumor (black). Reproduced from [12], copyright 2016, with permission from Elsevier. (f) A single cut of a 3D model allowing the view on the intracardiac anatomic features. Reproduced from [29], copyright 2008, with permission from Elsevier. (Abbreviations: Ao-aorta, ASD-atrial septal defect, LA-left atrium, LAD-left anterior descending coronary artery, LPA-left pulmonary artery, LV-left ventricle, MV-mitral valve, MVA-mitral valve annulus, PA-pulmonary artery, PM-papillary muscles, RA-right ventricle, RAA-right atrial appendage, RPA-right pulmonary artery, SVC-superior vena cava, TV-tricuspid valve, VS-ventricular septum, VSD-ventricular septal defect.)
Download figure:
Standard image High-resolution imageModel printing for other cardiac surgeries
3D-printed models can be utilized for planning surgical management in patients with aortic diseases (figure 4(d)), cardiac valve diseases, cardiac tumors (figure 4(e)) [12], and in adults with mitral valvulopathy [27, 28], to assess the precise mechanism of regurgitation. In the process of pre-procedural planning, the left ventricular outflow tract can be printed for patients with aortic stenosis and candidates for transcatheter aortic valve replacement (TAVR). A prospective study has reported that two patients were diagnosed with large, complex cardiac tumors, where 3D printing technology was utilized to analyze the size of the tumor, its location, and expansion more precisely, allowing the pre-operative planning and decision making as well [27, 28]. In another study to facilitate the planning and execution of cardiac surgical procedures, Jacobs et al, created an anatomical 3D rapid prototyping (RPT) model of the heart and its components with altered geometry, including right ventricular tumor as well as left ventricular aneurysm for early identification of risks in patients with severe complications. However, this RPT model lacks the appropriate validation, and that can be improved over pre-operative planning [21]. Bio-derived 3D plastic models of healthy (figure 4(f)) [29] and pathologic mitral valve annuli resembling MA in texture and flexibility were generated to assess the clinical feasibility using echocardiographic data to assist the surgical education [30]. Similarly, Bury et al prepared a new 3D-printed left atrial appendage (LAA) exclusion device by the SLS process to overcome the risk factors such as atrial fibrillation (AF) and stroke during surgery. This appendage is safe and feasible in animals, however, clinically yet remain to be proved [31].
In a case, 3D printing facilitated the surgical planning of extremely rare benign cardiac schwannoma originated from the cardiac plexus, resolving the tumor resection under cardiopulmonary bypass. This resection resulted in tumor enucleation and supportive in optimizing the surgical approach [32]. Fascinatingly, Sodian et al explored the effect of RPT by performing the aortic valve replacement (AVR) through re-sternotomy due to symptomatic aortic valve stenosis in patients with previous coronary artery bypass grafting (CABG). In addition, the fabricated 3D heart models have been used in surgical planning in both pediatric and adult cardiac surgery for a particular span of time as a single-center of experience and aimed to develop the models for individual therapies and non-routine procedures [9, 28, 33].
Microscale fibers for biomimetic cardiac tissue
TE holds a great promise in generating micro-sized (micron to a millimeter-sized range) tissue constructs that can recreate the structure and function of the damaged tissues [34]. These microfibrous-like structures (table 2) properly integrate with the cells and have been shown to provide instructional cues to cells for a proper phenotype. Numerous microfabrication strategies have been applied to engineer the biomimetic tissue constructs [35].
Generating simple 2D or hollow organs is feasible with cell and supporting scaffold of a type by molding them into a pre-designed cast. However, molding-based fabrication is even challenging to accommodate multiple cell types along with the extracellular matrix (ECM) in 3D space to accomplish the tissue-mimicking patterns and associated spatial resolution. Nevertheless, the generation of the 3D architecture of the cardiac tissue patch, valve, and the vessel is also expedient in cardiac surgery [36].
Myocardial tissue
MI is a lethal cardiac complication, which inspired the TE strategy for the preparation of 3D-printed cardiac tissue constructs. The pathophysiology of MI states that the heart pumps blood inadequately in injured cardiac tissue, due to the irregularity in contractility of cardiac muscle (primarily cardiomyocytes), and thus results in the formation of a thick scar (due to activated fibroblasts). The decrease in cardiac output often results in ischemia and leads to death. More often, the cellular therapy is practiced to repair the damaged heart by implanting cells at the injured site [11]. However, the ability of the cells to survive, properly integrate with cardiomyocytes during implantation and upsurge the cardiac output signifies the effectiveness of this therapy. Thus, cellular therapy alone faces the difficulty in achieving the desired repair and regeneration of the tissue. One of the key motives associated with the lowest survival rate of the implanted cells is the harsh microenvironment in the heart [36, 37]. To overcome these limitations, various TE strategies and their combinations are being explored. 3D printing is one of them that has grasped the potential interest of researchers and surgeons by enabling the fabrication of dense 3D constructs at a defined geometry of random intricacy possessing homogeneously dispersed cells. Yeong et al, produced 3D structures at different geometries (micron to millimeter scale) using the SLS technique. A CO2 laser-equipped SLS technique sinters a specific pattern and builds-up the layer-by-layer structure of 3D constructs, based on pre-designed CAD models [38]. In this study, polycaprolactone (PCL) yielded ∼89% of strain-sintered structures with a surface roughness of ∼34 μm, the porosity of ∼48%, and a tensile stiffness in the range of ∼0.43 MPa. Viable myoblasts were evidenced in multinucleated myotubes, and further performance characterization of this construct for therapeutic purposes yet remain to be done. This AM method is not suitable to print the cells directly due to the high-temperature requirement for sintering process [11].
In another study, Gaetani et al constructed a model containing human cardiac-derived myocyte progenitor cells (hCMPCs) and RGD peptide-modified sodium alginate as the ECM (figure 5(a)) by pressure-based 3D extrusion [36]. This tissue construct promoted the in vitro differentiation of viable hCMPCs into cardiomyocyte-like cells, and subsequently allowed the migration of hCMPCs out of the construct. hCMPCs in this tissue construct are highly advantageous because they are already committed to the cardiac lineage and can certainly differentiate and proliferate in vitro. The cell performance, i.e., differentiation and migration, improved greater in the 3D-bioprinted construct than 2D structures with subsequent upregulation of the transcription factors (a homeoprotein (Nkx-2.5), (a MADS box-transcription factor (Mef-2c), a zinc-finger-containing transcription factor (GATA-4), and the sarcomeric protein TroponinT (TnT)), suggesting the therapeutic potential of this cell population that can integrate with the damaged heart tissue if implanted. Nevertheless, hCMPCs did not exhibit the striated phenotype of cardiomyocytes, signifying that other factors such as mechanical and chemical cues are required for in vitro cell differentiation [36, 37]. Similarly, polyurethane urea-based cardiac patch was prepared using laser-induced-forward-transfer (LIFT) cell printing technique applying human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs) in a defined pattern (figure 5(b)), which facilitated the significant functional recovery after MI inducing cardiac regeneration through increased vessel formation, i.e., angiogenesis at the edge of the infarction [39].
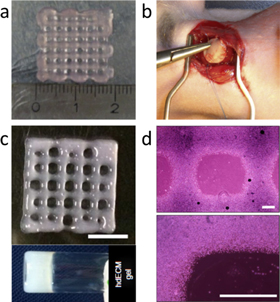
Figure 5. Various cardiac constructs at a micron to the millimeter-sized range. (a) Printed hCMPCs in 10% alginate by using tissue printing technology. Reproduced from [36], copyright 2012, with permission from Elsevier. (b) In vivo implantation of cardiac patch prepared by utilizing LIFT cell printing technique of hMSCs and HUVECs in a defined pattern on a polyurethane urea. Reproduced from [39], copyright 2011, with permission from Elsevier. (c) 3D-printed cardiac tissue using hdECM bio-ink, (scale bar: 5 mm) and rheological behavior of pre-gel, and (d) representative microscopic images of the hdECM construct (scale bar: 400 μm). Reprinted by permission from Macmillan Publishers Ltd: Nat. Commun [40], copyright 2014.
Download figure:
Standard image High-resolution imageA new 3D bioprinting opportunity from Cho's group was the extrusion-based printing using bio-inks for the production of dECM of whole organs (figure 5(c)) [40]. This dECM has been shown to provide instructional cues to cells for a proper phenotype. Similarly, bio-inks were generated from various tissues, including heart, cartilage, and fat to print the porous tissue-like structures. At this instance, rat myoblast cells were considered for the heart-derived bio-ink preparation due to limited availability of human cardiomyocytes (figure 5(d)). Eventually, this bio-ink increased the in vitro expression of cardiac-specific genes (Actn1 and Myh6) and cardiac myosin heavy chain (β-MHC) persistently than cells in other constructs such as collagen. However, the application of this top-down approach is limited due to difficulty in multiple cell patterning.
Undeniably, 3D printing is the most beneficial and convenient process for constructing porous materials to maintain the viability of cells. Despite its success and advantages, 3D printing of myocardium still has a couple of significant limitations. One of them is an inadequate source of human cardiac cells to be implanted, and the other is a limited printing resolution to build the complex structures. Utilization of cardiac progenitor cells and induced pluripotent stem cells (iPS cells) are more promising to solve the first issue. In addition, the 3D printing-based TE platform has addressed the other curb by building the high-resolution native-like scaffold using multiphoton-excited 3D printing that has significantly improved the recovery from ischemic myocardial injury [41].
Cardiac valves
In general, cardiac valve replacement surgery has been accomplished by placing either a biological or a prosthetic heart valve. More often, biological valves for a replacement surgery are prepared from either an allographic or xenographic tissue source [11, 42]. On the other hand, the prosthetic valves are mechanically robust with a longer lifetime than the biological valve. However, the application of this valve is limited because patients are required to rely on the intake of anticoagulants. The patients with biological valve replacement are not obliged to take anticoagulants for enduring but have to consume for a significantly shorter span, i.e., 10–20 years lesser compared to prosthetic valves [42]. To overcome these curbs, TE-based heart valves have been emerged as an effective alternative and are more promising because of using respective patient's cells for the valve preparation to avoid undesired immunogenic responses, no chronic drug usage, improved hemocompatibility, ability to repair itself, and even mature/age along with the patient (advantageous for younger patients). The desired qualities of any heart valve include holding minimal regurgitation of blood upstream, resulting in a minimal thrombogenic response, having a low transvalvular pressure gradient, and owing a high capacity to repair the damage [11].
3D printing technology-based TE has progressed the outcome of heart valves by fabricating them with patient-specific geometries in considering their spatial heterogeneity of valve's mechanical properties [20]. Herewith, we present a few interesting reports related to the engineered cardiac valves and its components through 3D printing. Recently, Blankstein and colleagues used 3D printing method assisted with pre-procedural cardiac CT for the better fabrication of a heart valve. They printed the individual components of aortic root complex possessing better mechanical properties for TAVR, and that has an ability to predict paravalvular aortic regurgitation (PAR). In the end, the resultant 3D-printed heart valve model was an exception, and patient-specific, demonstrating the ease of assessment of the interplay of implanted valves and aortic root (figures 6(a) and (b)) [20].
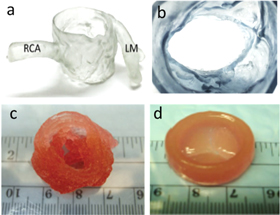
Figure 6. (a) 3D-printed models depicting the morphology to demonstrate the anatomical features of the aortic valve complex, and (b) reproduced the geometry of the annulus following the cardiac CT image. Reproduced from [20], copyright 2016, with permission from Elsevier. (c) 3D-printed aortic valve conduit prepared by utilizing aortic SMC and VIC within alginate/gelatin hydrogel. Reproduced from [42],John Wiley & Sons. © 2012 WILEY PERIODICALS, INC. (d) 3D-printed heart valve conduit with encapsulation of HAVIC within the leaflets to provide a functional de novo living valve replacement, reproduced from [43], copyright 2014, with permission from Elsevier. (Abbreviations: RCA-right coronary artery. LM-left main artery.)
Download figure:
Standard image High-resolution imageButcher and colleagues developed an extrusion-based 3D printer to generate the photo-cross-linkable hydrogels-based artificial valve with altered texture, i.e., rigid (∼75 kPa) hydrogel for the root, and another is soft (∼5 kPa) hydrogel for the leaflets [44]. The arbitrary geometries of the 3D artificial valve were printed easily ranging between 12 mm (average pediatric heart valve size) and 22 mm (average adult heart valve size) in diameter within 45 min at a high (93%) accuracy. Simultaneously, the ability to print two materials with distinct mechanical properties allowed the fabrication of heart valves' mimicking the altered stiffness in native heart valves between the leaflets and the root. This work has not been functionally tested yet, but this fabrication was a proof of principle. In addition, porcine aortic valve interstitial cells (PAVIC) seeded 3D-printed heart valve scaffolds were survived for up to three weeks demonstrating that fabricating the anatomical heterogeneous valve conduits, which facilitates cell engraftment. Similarly, a heart valve was directly fabricated with two types of cells, namely aortic root sinus smooth muscle cells (SMCs) and aortic valve leaflet interstitial cells (VICs), encapsulated into their root and leaflet regions (figure 6(c)) [42]. The alginate/gelatin mixture with cells exhibited better mechanical properties such as modulus, strength and peak strain higher compared to the acellular heart valve. In another study, 3D printing of HAVIC suspended methacrylate hydrogels (gelatin and hyaluronic acid (HA) mixture) (figure 6(d)) [43] was remodeled by depositing the ECM containing glycosaminoglycans and collagen. Moreover, these printed hydrogels would experience the changes in its stiffness in the phenotype-based hydrogel, where softer hydrogels induced the fibroblastic behavior to the cells.
Remarkably, Philippe et al created a simplified new in-silico 3D model of a human heart by an RPT method for transapical AVR during surgical training procedures. This was realized, life-size translucent set-up for training, transapical valved-stent delivery [45]. Also, a new approach for fabricating the custom-made heart valve using the SLS-based 3D printer from resorbable polymers with characteristic features, demonstrating that the approach can be implemented to both printing and cell seeding process, which is enough to support the complete functionality [46].
Above deliberated facts demonstrate that 3D printing is predominantly appropriate for developing heart valves due to ease of addressing the issues like complex geometry, heterogeneity in stiffness by incorporating multiple print heads, and the relatively lower contribution of cellular activity. In addition, this technique does not involve complex differentiation pathways and highly-ordered vascular networks for the development of a heart valve. However, comprehensive testing of TE-based 3D-printed heart valves yet remain to be done to improve the functionality.
Coronary arteries
The coronary artery impairment cases have risen to more than 16 million adults in the United States (US), accounting one-third of deaths annually regardless of improvement in various therapeutic strategies' [11]. The symptoms of such impairment are managed by considering its phase of seriousness, following, altered daily regime, therapeutic approach, and CABG surgery eventually [47]. CABG is recommended clinically (0.4 million cases annually in the US) for complex multi-vessel coronary artery disease involving diversion of blood around a damaged artery utilizing harvested alternative grafts. Typically, one or more conduits from internal thoracic arteries, radial arteries, or saphenous veins are harvested for an additional blood supply source to the distal coronary arteries congested by stenosis. CABG significantly improves the disease impairment for the patient survival. However, approximately one in every three patients are not eligible due to lack of appropriate autologous vessels. Despite the availability of site for surgical progress, CABG still faces several drawbacks such as poor long-term patency, accidental damage during harvest, and illness at the donor site after surgery [11, 33, 48].
Artificial coronary bypass grafts have garnered the interest of researchers and are expected to fulfill the unmet CABG needs mentioned above. A typical artificial vascular graft should ideally be biocompatible, durable, and anti-thrombogenic in nature, possessing similar compliance and density to native vessels [33, 49]. In the beginning, expanded polytetrafluoroethylene (ePTFE (Gortex)) and woven polyethylene terephthalate (PET (Dacron)) were used to synthesize artificial coronary grafts successfully to the aorta and peripheral vascular territories, however, they have failed to make the low-flow grafts with a narrow diameter. The poor outcome of these grafts is due to their undesirable biochemical properties yielding enormous activated inflammatory responses, causing poor patency because of thrombogenesis and improper mechanical characteristics such as lack the flexibility of polymers to stay in the proximity of the heart yielding complex geometry and cyclic deformation [33, 49, 50].
Furthermore, the advancement of artificial grafts has been explored using various polymeric constructs such as TE-based polyurethane vascular grafts to overcome the above-mentioned limitations. Despite the improvement in its mechanical properties, polyurethane vascular grafts have resulted in higher rates of thrombosis and infection and aneurysm formation [50, 51]. Vascular TE has been hoping for preparing non-thrombogenic endothelialized artificial coronary bypass grafts possessing a comparable native heart biomechanical properties with lively conduits for restoring hemodynamic function. These artificial TE grafts mimicking blood vessels can be engineered in two ways, one of them is a cell-sheet-based graft, which involves the cultured cells molded into a sheet that results in a tubular conduit mimicking the media and adventitia of an artery. Another approach is a scaffold-based graft, i.e., using natural, synthetic biomaterials, or decellularized matrix as scaffolds for backing cell attachment and their proliferation in vitro during the tissue development. Despite the exertions in producing artificial coronary bypass grafts, few of them have matched the long-term performance of autologous grafts. However, none of them is yet commercially available for clinical use [47].
The 3D printing technique has emerged as an effective alternative to fulfill the clinical requirements of utilizing the biocompatible 3D-printed-hydrogels using fibroblasts, differentiated endothelial cells, and SMCs, or hematopoietic and MSCs. This TE approach has also enabled by endorsing the patient-specific geometry grafts and printing them using various biomaterials. However, specific coronary bypass grafts yet to be printed through this technique directly. Currently, research is mostly focused on the generation of different vascular models in vitro and the inner lining of endothelial cells for the microvascular network formation to investigate the microenvironment of the engineered tissue [33, 48].
Recently, most of the studies were focused on fabricating 3D-printed vessels through a sacrificial template method. Khademhosseini and co-workers [52] reported the use of bioprinted agarose microfibers as templates to generate hollow vascular networks in photo-cross-linkable methacryloyl gelatin (GelMA) hydrogel constructs (figures 7(a) and (b)). These bioprinted agarose microfibers were easily extracted from the cross-linked hydrogels manually or by using a mild vacuum. Consequently, the acquired perfusable vascular networks were further coated with a layer of endothelial cells, at which, the assorted experiments have demonstrated that these networks gained functionality in improving mass transport, cellular viability, and their differentiation within the cell-laden tissue constructs. Recently, the similar group reported a strategy, which is capable of generating highly organized perfusable vessel structures [35] using a simple, one-step printing technique based on a multi-layered coaxial nozzle system. A blend of HUVECs, MSCs, alginate, GelMA, and poly(ethylene glycol)-tetra-acrylate (PEGTA) (figure 7(c)) was used as the functional bio-ink designed to flow in the outer needle of a multi-layer-print, while the calcium chloride solution flowed within the inner needle as well as ambient vapor to cross-link the extruded tubular structures [35]. The vascular cells delivered from the outer needle have grown gradually during a period of 21 days to form a confluent layer of endothelium in the hollow wall of the microfibers, leading to the formation of bio-relevant, highly organized, perfusable vessels. In another study, Lewis and colleagues developed an aqueous fugitive ink composed of Pluronic F127 for printing the multi-layer microfibrous networks, which are subsequently embedded in the hydrogel matrices. The entire constructs were then cooled down to 4 °C to liquefy the printed polymeric microfibers and led to the formation of microchannels within the matrices (figures 7(d) and (e)) [53].
Figure 7. (a) 3D-bioprinted branching of agarose templates (green) embedded in a methacryloyl gelatin (GelMA) hydrogel construct, and (b) resulting 3D branching network, microchannels perfused with a fluorescent microbead suspension (pink) (scale bar: 3 mm, microchannels have 500 μm in diameter). Reproduced from [52], copyright 2014, with permission from Royal Society of Chemistry. (c) Fluorescence micrographs of the bioprinted tubular constructs composed of 10 layers using a specially designed cell-responsive bio-ink consisting of GelMA, sodium alginate, and 4-arm PEGTA by multi-layered coaxial extrusion system (scale bar: 2 mm). Reproduced from [35], copyright 2016, with permission from Elsevier. (d) Bright field microscopy image of the 3D-printed 10T1/2 fibroblast-laden GelMA, fugitive, and green fluorescent protein (GFP) human neonatal dermal fibroblasts (HNDF)-laden GelMA, inks construct (scale bar: 500 μm), and (e) image acquired during fugitive ink evacuation (scale bar: 5 mm). Reproduced from [53] John Wiley & Sons.© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution imageInterestingly, the significant results from various studies demonstrated the potential of generating small-sized tubular structures using 3D printing method. However, several challenges yet remain to accomplish the microvascular 3D printing to prepare an ideal artificial coronary bypass graft. Indeed, it is tough to print the scaffold and subsequent cell seeding to mimic such layered structures of autologous blood vessels containing different cellular components with excellent mechanical properties such as stiffness. TE-based 3D printing of microscale vasculature also remains unclear regarding the cell seeding in the printed tubular scaffolds. Eventually, providing the physiological conditions mimicking the environment to cells within the coronary bypass graft is challenging. Therefore, future TE-based vascular grafts may address the above-discussed challenges by creating both micro- as well as macrovascular structures concomitantly providing the physiological environment mimicking conditions similar to Vasa vasorum [1, 54].
Nanoscale components for functional improvement
The integration of nanotechnology to a fabrication method such as 3D printing offers enormous potential and tremendous opportunities for the functional improvement and structural restoration of ECT and its efficient therapeutic approaches. In native cardiac tissue, Purkinje fibers exert conductivity within the whole heart, that can propagate the electrical communication among adjacent cells. However, the current ECT lacks the conductivity. The present state-of-the-art fabrication of methodologies for synthesizing nanoconstructs and their unique features includes advantages, limitations, resolutions, along with the process constraints, may progress the functional maturation and cardiogenesis [55].
In recent times, ECTs have grabbed the attention of researchers in improving the electrical signals to enhance the cellular excitability of both cardiomyocyte and neuron. This conductivity endorses a series of steps involving the cell differentiation and their long-term survival. These advanced biomaterials have played a crucial role in tissue constructs and bionic devices (i.e., cardiac pacemakers) efficacy in the improvement of various cardiac disorders. Therefore, these electrically active components can be promoted as 3D-printed objects, which mimic the electrophysiological environment of the native myocardium [56]. Corresponding advances in techniques and materials used are required to investigate the potential of 3D printing of multifunctional nanocomposites [3]. Similarly, future materials based on these strategies continue to drive the advancement of disease treatment by targeting cardioprotective drugs, TE to replace defective valves, patches for damaged heart muscle and imaging. TE-based nanocomposites and their entrenchment in hydrogels have been successful, but they are not within the scope of the present review, and thus not discussed here. Several review articles have been focused on cardiac TE application [57–61]. Herewith, we present a brief overview on nanoconstructs such as carbon nanotubes (CNTs), gold nanoparticles (GNP), graphene oxide (GO), mesoporous silica nanoparticles (MSNs) and polymeric carriers (PCs), and others, essential in cardiac TE and regenerative medicine. The utilization of nanomaterials in 3D printing may create a scope in the advancement of the field that may revolutionize the treatment options in patients at the end-stage of heart failure to enhance the viability and function of cardiomyocytes to combat the heart failure.
CNTs are one of the most popular TE materials, that efficiently progresses the cardiogenesis through the efficient propagation of electrical signals [62]. CNT and its functionalized nanoconstructs [63] have been at the forefront as ideal materials to reform the regenerative medicine and have also gathered the fascinating interest of researchers in the advancement of many devices with significant properties such as biocompatibility and others [64]. The utilization of CNTs has resulted in the improvement of electrical behavior and interfacing of myocyte along with the changes in the scaffold morphology, proliferation, and maturation. CNTs interaction with cardiomyocytes splendidly enables the substantial augmentation in electrical conductivity when used as nano-based polymeric devices supporting cell proliferation and differentiation [65]. The synchronized electrical activity directly represents the practical strategies of the cardiac repair [64, 66]. Yoon and Kim, colleagues, fabricated the 3D printing structures of PCL-CNT composite scaffolds, which have shown potential in cardiac TE. CNT alignment reinforced the polymeric chains resulting in the enhancement of crystallinity of the PCL matrix [67]. Dispersed CNTs were also involved in the induction of gene-silencing for cardiac repair, i.e., by increasing the connexin-43 expression using non-genetic engineering methodology [66].
GNPs are capable of promoting the cell organization and the cardiac function improvement effectively [68]. Typically, the 3D architecture of cardiac tissue patches aims to improve the unique microenvironment composed of various size elements that mimic the native cardiac matrix to a rapid transfer of the electrical wave. Few instances, it lacks the electrical coupling between the adjacent cells, which makes the treatment vulnerable. Dvir et al addressed this curb by depositing GNP onto the decellularized tissue matrix to accomplish the engineered functional cardiac patch preparation for treating MI. These hybrid patches resulted in superior function, better elongation, and strong contraction force featuring organized connexin-43 electrical coupling proteins [55]. Similarly, coiled electro-spun fibers incorporated GNP resembling the coiled perimysial fibers of the native heart improved the ability of contraction and relaxation of the heart muscle, by promoting the electrical coupling [69, 70].
GO, the other promising class of carbon-based candidates, which has been fascinatingly utilized towards cardiac TE. The inherent properties of graphene interfere with living cells as well as efficient electrical stimulation to mimic the highly organized 3D complex architectures of cardiac ECM [71]. In addition to the innovative nanomaterials discussed above, other materials such as MSNs [72, 73], PCs [74] were used for various reasons in cardiac TE to induce/regulate the cardiomyocyte differentiation.
Cardiac drug delivery
Post-surgical procedures involving various drug administration processes are crucial for heart-related ailments. Recently, 3D printing has garnered the interest of researchers for the development of different delivery systems administered through various procedures. Jonathan et al compiled the application of 3D printing process for the preparation of various dosage forms intended for treating various ailments [75]. Interestingly, Polypill for cardiovascular treatment was formulated by an extrusion-based printing method incorporating an osmotic pump for the sustained drug release characteristics. This approach was successful in eliminating the complicated dosage regimens [76]. However, the patient compliance limits its administration procedure and also the reduction of the pill size is challenging.
Numerous multifunctional drug delivery vehicles suitable for cardiac regeneration, and electrical conductivity improvement have been developed for potential therapeutics. Though discussing this section here is out of the scope of the article, we give a brief note on cardiac drug delivery using other nanoparticle-based approaches. The advancement in cardiac drug delivery has evidenced the development of various innovative drug delivery carriers. One of the important classes of delivery includes the utilization of biodegradable polymeric micro- and nanospheres for the delivery of various cardioprotective drugs (such as Carvedilol) and growth factors (such as VEGF) with higher clinical efficacy [74, 77]. On the other hand, the polymeric hydrogel (poly-(ethylene glycol) -mediated and self-assembling peptide carriers such as VEGF microparticles for cell and growth factor delivery were formulated to improve the mechanical properties or impart specific functionalities [78, 79]. Other approaches include the utilization of one of the most advantageous inorganic nanocarriers for controlled release systems, i.e., MSNs for the delivery of 5-azacytidine and ascorbic acid for the regulation of cardiomyocytes differentiation during regeneration [72, 73]. The potential nanoparticle-based delivery carriers are mostly administered through various routes of systemic/injectable administration, however, to achieve the localized delivery with minimal adverse effects, direct intramyocardial injection is ideal [79]. These multifunctional systems, aim to heal and drive the ailment faster towards effective cardiac regeneration.
Table 1. Fabrication of macro sized components for cardiovascular system prepared using 3D printing technologies.
| Components | Manufacturing process | Materials | Size | Printing time | Outcome | Reference |
|---|---|---|---|---|---|---|
| Annuloplasty ring | SL | Accura® 60 epoxy resin | ∼29 mm | — | Personalized development of an annuloplasty ring to treat mitral insufficiency | [19] |
| Aorta | IJP | Porcelain, starch/cellulose powder | >10 cm | 5–7 h | Model suitable for positioning the valve deeper into the aortic annulus | [28] |
| SL | Liquid photopolymer | >2 cm | 6–12 h | Model suitable to assess coarctation of the anatomical features | [80] | |
| STL | Urethane resin for infiltration | >10 cm (wall thickness- 2–3 mm) | ∼24 h | Model suitable for better pre-operative planning and decision making of FET procedure | [27] | |
| Aortic root complex | STL | Flexible resin | 23–29 mm, (wall thickness- 2 mm) | ∼3.5 h | These models were helpful for TAVR planning and to determine the ability of PAR | [20] |
| Open-source RPT system (Fab@Home) | Silicone-based material | 13.2 × 4.4 × 2.8 mm | ∼40 h | This physical 3D model was created in a single-step procedure by reducing the complexity and time of creation | [81] | |
| Atrial appendage (left) | SLS | Polyamide powder-PA2200 | 40 mm | — | This model was helpful in elective cardiac operations to prevent LAA thrombus | [31] |
| Atrial septal defect | FDM | ABS polymer | 27 × 24 mm | ∼60 h | This model was helpful in the analysis of procedural failures and adverse events | [13] |
| Atrial septal occluder device | FDM | ABS polymer | 10 mm | ∼60 h | This model was helpful in successful percutaneous closure of the periprosthetic defect | [13] |
| Coronary artery bypass graft and the sternum | IJP | Starch/cellulose powder | >5 cm | 5–7 h | This model was helpful for optimal surgical planning, intraoperative decision making and reopening the sternum | [28] |
| Heart model | IJP | Starch/cellulose powder | >10 cm | 5–7 h | This model was helpful in assisting in perioperative planning and procedure simulation | [28] |
| Plaster | Layer thickness 0.5–1.0 mm (512 × 512 matrix) | 3–8 h | Model facilitating the view of individual cardiac structures of patients with an aneurysm and the left ventricle residual-volume | [21] | ||
| 175 slices, each (0.7 × 0.7 × 0.9 mm) | ∼7 h | The spatial imagination of complex congenital cardiac abnormalities for subsequent surgical intervention is improved | [29] | |||
| SL/IJP | Liquid resin, polyurethane | — | — | Spatial relationships between anatomic structures can be represented precisely during pre-operative planning | [82] | |
| SL | UV-curable photopolymer resin | Wall thickness- 3 mm | — | Preparation for a percutaneous mitral annuloplasty using the mitralign percutaneous annuloplasty system | [83] | |
| FDM | ABS polymer | Layer thickness-100–180 μm | — | These 3D models were helpful for both myocardial and blood pool segmentation | [84] | |
| Open-source RPT system (Fab@Home) | Silicone-based material | — | ∼72 h | An excellent set-up for surgical training of transapical valved-stent delivery | [45] | |
| Intracardiac lesion | IJP | Starch/cellulose powder | >5 cm | 5–7 h | This rigid model was beneficial for decision making and intraoperative orientation in reconstructing intracardiac defects in pediatric patients | [28] |
| Mitral annuli | STL | Bio-derived plastic | Thickness- 1.75 mm | 15 min | This model was helpful in assisting with surgical education, planning, and decision making rapidly | [30] |
| Thoracic-aortic pseudoaneurysm | FDM | ABS polymer | 31 × 15 mm | ∼60 h | This model was helpful in pursuing thoracic-aortic pseudoaneurysm and ulcerations | [13] |
| Tumor (cardiac) | IJP | Starch/cellulose powder | >2 cm | 5–7 h | This model was helpful for exact anatomical understanding to identify the position and expansion of tumors' clearly | [28] |
| Vascular rings | SL | Liquid photopolymer | >2 cm | 6–12 h | These vascular ring models were helpful for surgical findings | [80] |
| Ventricular septal defect | FDM | ABS polymer | 12 mm | ∼60 h | This 3D model that defined the VSD anatomy and spatial orientation to surrounding structures | [13] |
| Y-shaped coronary stent | — | Wax material (VisiJet® Hi-Cast), PDMS | 15 × 3 mm | ∼72 h | The model was constructed based on invasive coronary angiography | [85] |
Abbreviations: acrylonitrile butadiene styrene (ABS), frozen elephant trunk (FET), fused deposition modeling (FDM), Iinkjet-printing (IJP), laser-based stereolithography (SL), left atrial appendage (LAA), paravalvular aortic regurgitation (PAR), polydimethylsiloxane (PDMS), selective laser sintering (SLS), stereolithography (STL), transcatheter aortic valve replacement (TAVR), ventricular septal defect (VSD). ('—' represents not determined.)
Table 2. Fabrication of micro-sized components for cardiovascular system prepared using 3D printing technologies.
| Components | Technology | Materials | Size | Outcome | Reference |
|---|---|---|---|---|---|
| Aortic valve conduits | Extrusion-based printing | Alginate, PEG-DA hydrogel | 12–22 mm | 3D hydrogel printing with controlled photo-crosslinking rapidly fabricated the anatomical heterogeneous valve conduits | [44] |
| Alginate/gelatin hydrogel | ∼2 mm | Anatomically complex, heterogeneously encapsulated aortic valve hydrogel conduits | [42] | ||
| Cell-laden dECM | Multi-head tissue/organ building system | PCL | 100–200 μm | Optimized microenvironment conducive for the growth of 3D structured cardiac tissue | [40] |
| hCMPCs | Tissue printing technology | Sodium alginate | Distance: 200 μm (final dimensions 2 × 2 cm) | Cardiac progenitor cells entrenched polymeric scaffolds for tissue regeneration | [36] |
| Heart valve | SLS | PGA-/PLA-based copolymers | >150 μm | A new 3D structured-valve resorbed by the human body in the course of the growth process | [46] |
| Open-source RPT system (Fab@HomeTM) | Me-HA, GelMA, HAVIC | 5 × 5 × 1.5 mm (L × W × H) | Living valve scaffolds through bioprinting accelerated the understanding of physiological valve cell interactions | [43] | |
| Mitral valve | SL | PAA | Thickness: 0.641–1.62 mm | Polymeric models created from echocardiographic data enhanced the spatial perception of cardiac anatomy and pathology | [86] |
| STL | ABS plastic | Thickness: 0.25 mm | To guide surgical therapy for mitral valve disease (ischemic mitral regurgitation and myxomatous degeneration) | [87] | |
| Vascular vessels | Template micro molding | GelMA, SPELA, PEGDMA, PEG-DA | 500 μm | To demonstrate the functionality of the fabricated vascular networks in improving mass transport | [52] |
| Extrusion-based printing | Pluronic F127, PEO-PPO-PEO | ∼200 μm | The highly scalable platform allows to produce vasculature, and multiple cell types are programmable placed within the ECM | [53] | |
| GelMA, sodium alginate, PEGTA | ∼500–1500 μm | A convenient and versatile 3D bioprinting strategy for direct deposition of cell-laden perfusable vascular constructs | [35] |
Abbreviations: acrylonitrile butadiene styrene (ABS), decellularized-extracellular matrix (dECM), human aortic valvular interstitial cells (HAVIC), human cardiac-derived cardiomyocyte progenitor cells (hCMPCs), human umbilical vein endothelial cells (HUVECs), laser-based stereolithography (SL), methacrylated-gelatin (GelMA), methacrylated-hyaluronic acid (Me-HA), polyacrylic acid (PAA), polycaprolactone (PCL), poly-(ethylene glycol)-diacrylate (PEG-DA), poly-(ethylene glycol) dimethacrylate (PEGDMA), poly-(ethylene glycol)-tetra-acrylate (PEGTA), poly-(ethylene oxide) (PEO), polyglycolic acid (PGA), polylactic acid (PLA), poly-(propylene oxide) (PPO), selective laser sintering (SLS), star poly-(ethylene glycol-co-lactide) acrylate (SPELA), stereolithography (STL).
Perspective
Surgical bioprinting models are extremely appreciated and helpful in assisting such complicated procedures. However, a clinical guide by 3D-printed model needs further improvement in the resolution, speed, and modulation. The future potential of 3D-component fabrication is anticipated to use the dynamic guidance at every stage right from the pre-operation, while operation to post-operation procedures. Therefore, we can get rich information from pathology to judge the surgical complications. Despite the pros- and cons-associated, contemporary 3D printers will undeniably advance in their development and printing approach. The cardiac TE curbs have no effect on model 3D fabrication. However, they limit the processing during the human stem cell progression for efficient conditioning of cardiac tissues. 3D printing of various nanoparticles impregnated hydrogels will undoubtedly create a significant impact in cardiovascular TE. Similarly, excellent materials continue to drive the improvement of cardioprotective therapy by targeting drugs, TE to replace defective valves, patches for damaged heart muscle and imaging, and others. Nevertheless, progress in all the areas of research involving biologists, surgeons, engineers, as well as material scientists and collaboration between them will synergistically expedite the entire 3D printing field.
Acknowledgments
AZC acknowledges financial support from National Natural Science Foundation of China (U1605225, 31570974 and 31470927), Public Science and Technology Research Funds Projects of Ocean (201505029) and Promotion Program for Young and Middle-aged Teachers in Science and Technology Research of Huaqiao University (ZQN-PY107). RKK acknowledges financial support from Huaqiao University (Project No. 16BS803). KZ acknowledges National Natural Science Foundation of China (81301312), 'Chen Guang' project funded by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (Project No. 14CG06), and Shanghai Pujiang Program (Project No. 17PJ1401500).
Conflict of interest
The authors state that this article content has no conflict of interest.