Abstract
Scaffold development approaches using autologous sources for tissue repair are of great importance in obtaining bio-active/-compatible constructs. Platelet-rich plasma (PRP) containing various growth factors and platelet lysate (PL) derived from PRP are autologous products that have the potential to accelerate the tissue repair response by inducing a transient inflammatory event. Considering the regenerative capacity of PRP and PL, PRP/PL-based scaffolds are thought to hold great promise for tissue engineering as a natural source of autologous growth factors and a provider of mechanical support for cells. Here, a bio-mineralized PRP-based scaffold was developed using oxidized dextran (OD) and evaluated for future application in bone tissue engineering. Prepared PL/OD scaffolds were incubated in simulated body fluid (SBF) for 7, 14 and 21 d periods. Mineralized PL/OD scaffolds were characterized using Fourier transform infrared spectroscopy, x-ray diffraction spectroscopy, scanning electron microscopy (SEM), thermogravimetric analysis, porosity and compression tests. SEM and energy-dispersive x-ray spectroscopy analyses revealed mineral accumulation on the PL/OD scaffold as a result of SBF incubation. In vitro cytotoxicity and in vitro hemolysis tests revealed that the scaffolds were non-toxic and hemocompatible. Additionally, human osteoblasts (hOBs) exhibited good attachment and spreading behavior on the scaffolds and maintained their viability throughout the culture period. The alkaline phosphatase activity assay and calcium release results revealed that PL/OD scaffolds preserved the osteogenic properties of hOBs. Overall, findings suggest that mineralized PL/OD scaffold may be a promising scaffold for bone tissue engineering.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Bone is a specialized and vascularized tissue with a complex and organized composition possessing high regenerative capacity [1]. Despite the fact that bone remodeling remains in force during the lifetime, successful regeneration may not always occur in large bone defects [2]. In such cases, bone grafts emerge as viable bone substitute materials. Over the years, different types of grafts have been used to meet the need for bone replacement arising from a variety of reasons, such as fractures, osteoporosis, infections, trauma, injuries, and tumors. Bone grafts are desired to promote differentiation of progenitor cells into an osteoblastic lineage, to support bone growth and regeneration, and also to have the capacity to integrate with the surrounding bone tissue. Although different types of bone substitutes, including autografts, allografts or xenografts, have been reported in the literature [3–6], there are still many limitations and challenges in providing functional bone grafts, and therefore research continues for new bone substitutes that meet clinical needs.
Platelet-rich plasma (PRP) is a high concentration of platelets obtained from autologous whole blood by double centrifugation [7, 8]. As PRP contains a high concentration of autologous growth factors responsible for wound healing and tissue regeneration [9], its use has extended over the years from orthopedic, oral and maxillofacial surgery to plastic surgery [10]. The advantages of autologous PRP are the absence of immunogenicity and the risk of disease transmission, rapid and easy preparation, and low cost [11]. Bone formation process (chemotaxis, cell migration, proliferation and differentiation) is regulated by growth factors, many of them present in the PRP. For example, some of the chemotactic factors, such as platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1) and collagen, stimulate the migration of mesenchymal stem cells (MSCs) and progenitor cells to the defect site to promote bone regeneration. PDGF and TGF-β1 induce cell proliferation, as well. TGF-β1 is involved in osteogenic differentiation of MSCs. Osteoblastic differentiation is controlled by insulin-like growth factor and bone morphogenetic protein. Vascular endothelial growth factor (VEGF) stimulates the angiogenesis process, which is essential for bone formation and remodeling [12]. As a result, the availability of such growth factors capable of the basic biological functions in PRP has led to a significant increase in its use for bone tissue regeneration [13]. PRP has been used for tissue regeneration by local injection or in combination with biomaterials. There are a number of studies showing effects of PRP on bone tissue regeneration and repair. A recent study of relevance to bone repair reported that the regeneration effect of platelet lysate (PL) combined with allograft was better compared to that of the allograft alone [14]. Qiu et al. (2021) reported that calcium phosphate cement (CPC)/PL-based scaffold loaded with doxycycline (DOX) antibiotic had a promising effect for both the treatment and prevention of bone infection and enhancement of bone regeneration [15]. It has been recently demonstrated that methacrylated PL-based nanocomposite developed for bone repair and regeneration capable of releasing growth factor enhanced the osteogenic differentiation of MSCs [16]. In one study, three-dimensional (3D) hierarchical osteogenic scaffolds were prepared by assembling PL and polysaccharides layer-by-layer (LbL) and shaped into fibrils by freeze-drying. The results indicated that the incorporation of PL using LbL assembling and freeze-drying induced the differentiation of human adipose stem cells (hASCs) with only ten tetralayers [17]. Ribeiro et al [18] developed bone biomimetic cryogel scaffolds based on PL and biomineralized cellulose nanocrystals. The results showed that mineralized nanocomposite cryogel scaffolds promoted osteogenesis and angiogenesis. This study has provided a new perspective on the production of PRP cryogel scaffolds for bone tissue engineering.
In a previous study, we used oxidized dextran (OD) as a chemical cross-linking agent for the first time to create PRP cryogel scaffolds [19]. Unlike toxic crosslinkers such as glutaraldehyde, OD with aldehyde groups has attracted attention in recent years as a biocompatible, biodegradable and hydrophilic polysaccharide for the development of tissue engineering scaffolds. On the other hand, dextran can also be chemically modified to incorporate amine, sulfate groups or integrin-specific molecules on the polysaccharide backbone. The polysaccharide acting as a cross-linker can increase cell adhesion properties [20] while making the PRP scaffold resemble the native extracellular matrix (ECM) [21].
In this study, we developed a biomineralized PL/OD cryogel scaffold without the need for any chemical initiator for prospective use in bone tissue engineering and tested it with human osteoblasts (hOBs). Biomaterial characterization studies were comprehensively carried out to evaluate the physical and chemical properties. Additionally, cell culture analyses were conducted to evaluate the cell attachment and proliferation on the PRP-based scaffolds, and examine their osteogenic potential. The findings suggest that biomimetically mineralized PRP/OD-based macroporous cryogel may have the potential to be a xeno-free bioactive 3D scaffold for prospective bone repair applications.
2. Materials and methods
2.1. Materials
Sodium metaperiodate powder (99.8%–100.3%) and dextran (Mw 20 kDa) were supplied from Alfa Aesar (Kandel, Germany). Propylene glycol was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco's modified eagle medium (DMEM), L-glutamine and penicillin/streptomycin were supplied by Biological Industries (Kibbutz Beit-Haemek, Israel). All other reagents were purchased from Sigma-Aldrich and were of analytical grade.
2.2. Synthesis of OD
OD was synthesized according to a method described elsewhere [22]. Ten grams of dextran (0.0618 mol) (Mw: 20 kDa) was dissolved in 50 ml of distilled water. Fifty ml of 26.4% (w/v) sodium periodate (0.0618 mol) solution was slowly added to the dextran solution. The mixture was stirred at room temperature in the dark for 1 h. Then, propylene glycol (4.53 ml) with the same molar ratio as sodium periodate was added to the mixture to stop the oxidation reaction. The OD solution was dialyzed against distilled water for 1 week at 4 °C. After dialysis, the OD solution was lyophilized and stored at 4 °C until use.
2.3. Preparation of PL
PL was obtained by freeze-thawing PRP samples obtained from the AU Ibni Sina Hospital Blood Center (AU ISHBC # 13-625-16). PRP samples from five healthy donors were pooled to reduce inter-donor variability [23]. Pooled PRP was kept at −80 °C for 24 h and then incubated for 1 h in a 37 °C water bath. This process was repeated three times. The pooled PRP sample was then centrifuged at 2000 g for 10 min to remove cellular debris. The resulting supernatant was filtered through a 0.22 µm filter and stored at −80 °C until use.
2.4. Preparation and in vitro mineralization of PL/OD scaffolds
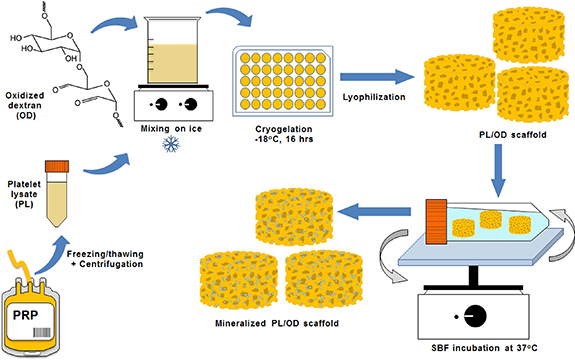
PL/OD scaffolds were produced as described previously [19]. Figure 1 shows a schematic representation of the preparation and in vitro mineralization of PL/OD scaffolds. Briefly, 2% OD solution was prepared in PL and stirred on an ice bath until completely dissolved. The final PL/OD solution was immediately transferred to the wells of the multi-well culture dish and placed in an ultrafreezer at −18 °C for 16 h. PL/OD scaffolds (Ɵ = 10 mm, h = 8 mm) were lyophilized for 24 h and then stored at +4 °C. For biomineralization, PL/OD scaffolds were immersed in the simulated body fluid (SBF) solution prepared according to the recipe reported by Kokubo et al [24], with minor modification (table 1). Lyophilized PL/OD scaffolds were sterilized by immersion in 70% ethanol solution and then washed three times with sterile PBS. Then the sterilized scaffolds were incubated in 1.5X SBF solution at 37 °C for different periods (7, 14 and 21 d). After incubation, the biomineralized scaffolds were removed from SBF, washed gently with distilled water, frozen at −80 °C, and freeze-dried for 24 h.
Figure 1. Schematic representation of the preparation and in vitro mineralization of PL/OD scaffolds.
Download figure:
Standard image High-resolution imageTable 1. Chemicals used in the preparation of 1.5X SBF (pH 7.4).
| Reagents | Amounts in 1000 ml |
|---|---|
| NaCl | 11.994 g |
| NaHCO3 | 0.525 g |
| KCl | 0.336 g |
| K2HPO4 | 0.261 g |
| MgCl2 | 0.214 g |
| 1 M HCl | 60 ml |
| CaCl2 | 0.417 g |
| Na2SO4 | 0.107 g |
| (CH2OH)3CNH2 | 9.086 g |
2.5. Scanning electron microscopy (SEM) analysis
The general morphology, pore structure, and mineral deposition of the PL/OD scaffolds were evaluated by SEM analysis. For analysis, lyophilized scaffolds were placed on an aluminum stub, sputter-coated with gold-palladium under vacuum and examined using a Zeiss Evo-40 model instrument (Jena, Germany). Energy-dispersive x-ray spectroscopy (EDS) analysis was also performed to determine the amount of calcium in the scaffolds.
2.6. Fourier transform infrared spectroscopy (FTIR) analysis
The chemical characterization of PL, OD and PL/OD scaffolds was performed by using a Spotlight 400 model instrument (PerkinElmer, Waltham, MA, USA). The lyophilized samples were powdered and analyzed at the range of 4000–400 cm−1 with a resolution of 4 cm−1.
2.7. X-ray diffraction (XRD) analysis
Phase analysis of the scaffolds was performed with a Miniflex model x-ray powder diffractometer (Rigaku, Tokyo, Japan). Before the procedure, the scaffolds were finely ground, homogenized and diffraction patterns were recorded at a scanning speed of 2° min−1 in the range of 10°–70°.
2.8. Density and porosity measurement of the scaffolds
The pore size distribution analysis of the scaffolds was performed using a Quantachrome Poremaster 60 model mercury porosimeter (Anton Paar, Graz, Austria) under low pressure conditions (0–50 psi). In addition, the fluid displacement method (Archimedes' principle) was used to measure the porosity and density of the scaffolds [25]. For this, the initial weights (Wi ) of the lyophilized scaffolds were measured, and then the scaffolds were immersed in 5 ml of pure ethanol to allow ethanol to enter the pores. The final weights (Wf ) were determined after ethanol immersion, and the density and porosity of the scaffolds were calculated according to equations (1) and (2),
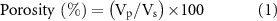
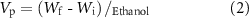
where, Vp is the total pore volume, Vs is the volume of the scaffold and ρethanol is the density of ethanol.
2.9. Water uptake
The water uptake capacity (WUC) of the scaffolds was determined by the general gravimetric method. The dry scaffolds were weighed and their initial weights were recorded as M0. Then, they were incubated at 37 °C for 24 h in PBS buffer (pH 7.4). After incubation, the scaffolds were removed from PBS. After removing the excess buffer from the surface, the wet scaffolds were weighed and recorded as M1. The WUC of the scaffolds were calculated using equation (3),
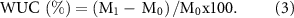
2.10. Mechanical test
Compression test was carried out to examine the mechanical properties of PL/OD scaffolds. For this, non-mineralized and mineralized scaffolds were incubated for 24 h in a PBS buffer (pH 7.4) at 37 °C. At the end of 24 h, a compression test was applied to the scaffolds using the AGS-X model mechanical testing device (Shimadzu, Tokyo, Japan) with 500 N load cell at a test speed of 1 mm min−1. The elastic modulus for all scaffolds was calculated from the initial slope of the stress-strain curves obtained by the compression test.
2.11. Thermogravimetric analysis (TGA)
The thermal stability of PL/OD scaffolds was evaluated using the Pyris 1 TGA model thermogravimetric analyzer (PerkinElmer) at a heating rate of 10 °C min-1 from 25 °C to 600 °C under nitrogen environment. Mass changes resulting from heating applied to the PL/OD scaffolds were determined by thermal gravimetric (TG) analysis. Differential TG (DTG) curves of PL/OD scaffolds were derived from thermogravimetric data.
2.12. In vitro cytotoxicity testing
In vitro cytotoxicity testing was performed according to the ISO 10993-5 standard for the biological evaluation of medical devices. For this purpose, the scaffolds were sterilized with 70% ethanol, and washed with sterile PBS to completely remove the alcohol. The scaffolds were then placed in a 24-well culture plate and incubated in proliferation medium (PM) [DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine] at 37 °C for 24 h. At the end of this period, the scaffolds were removed from the medium and the scaffold extracts were obtained. On the other side, hOBs (ATCC® CRL11372TM, Manassas, VA) were seeded in a 12-well plate at a density of 2 × 105 cells per well, and cultured in PM until they reached 70% confluence. Thereafter, hOBs were cultured with scaffold extracts for 48 h. Cells cultured with phenol solution (1%) were used as positive control (PC), and cells cultured with PM were used as negative control (NC). The percentage of cell viability of all groups was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [26].
2.13. In vitro hemolysis testing
In vitro blood compatibility of scaffolds was analyzed according to ISO 10993-4 standard. The scaffolds were first washed with PBS and incubated in 1 ml of PBS for 30 min at 37 °C with gentle shaking. One ml of distilled water was used as PC and 1 ml of PBS as NC. Two ml of fresh human whole blood (AU ISHBC # 13-625-16, and AU ISHBC # E-85422) was then diluted with 2.5 ml of PBS. After 30 min of incubation, 20 µl of diluted blood was added to the scaffolds and control groups, allowing for incubation at 37 °C for 1 h. After incubation, the samples were centrifuged at 520 g for 10 min. The absorbance (A) values of the supernatants were measured at 545 nm using a SpectraMax M5 model microplate reader (Molecular Devices, Sunnyvale, CA, USA). Percentage of hemolysis was calculated using equation (4),
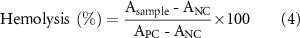
where Asample, APC and ANC correspond to the absorbance values of the sample, PC, and NC, respectively.
2.14. Cell proliferation (alamarBlue™) assay
At predetermined time intervals (days 3, 7, 10, 14 and 21), the viability of hOBs seeded on PL/OD scaffolds was evaluated using the alamarBlue™ assay. For cell seeding, scaffolds (Ɵ = 6 mm, h = 3 mm) were sterilized in 70% ethanol for 2 h and washed three times for 30 min with sterile PBS. After sterilization, the scaffolds were placed in a 48-well culture plate. The hOBs were harvested using trypsin/EDTA and suspended in PM. Thirty µl of cell suspension (containing 5 × 105 cells) was seeded on each scaffold and incubated for 40 min at 37 °C, 5% CO2 to allow the cells to adhere to the PL/OD scaffolds. Next, 1 ml of PM was added to each well and the cell-seeded scaffolds were cultured in PM for 21 d. Culture medium was collected at predetermined time periods, scaffolds rinsed three times with PBS. alamarBlue™ solution (10%) was added to the scaffolds and incubated at 37 °C for 150 min. After incubation, the fluorescence intensities of the samples were measured using a microplate reader at 560 nm excitation and 590 nm emission. Cell-free scaffold was used as the control.
2.15. SEM analysis of cell-seeded scaffolds
Adhesion, spreading, proliferation and morphology of hOBs seeded on PL/OD scaffolds were evaluated using SEM. At specified time points, samples fixed in glutaraldehyde were dehydrated through an ethanol series (50%–100%) and air dried. The dried samples were attached to stubs, coated with gold-palladium and viewed at different magnifications using a Zeiss Evo-40 model instrument. Elemental analysis of scaffold surfaces was also performed using EDS.
2.16. Osteogenic induction
The osteogenic potential of hOBs seeded on the PL/OD scaffold was examined by osteogenic induction experiments. For this, cells seeded on scaffolds were cultured with DMEM supplemented with 50 mg ml−1 ascorbic acid, 10 mM β-glycerophosphate and 10−8 M dexamethasone [27]. Cells on PL/OD scaffolds were cultured in osteogenic medium for 21 d. The amounts of calcium and ALP released from hOBs to the culture medium during osteogenic induction were analyzed using the QuantiChrom™ Calcium assay kit and the QuantiChrom™ Alkaline Phosphatase (ALP) assay kit (BioAssay Systems, CA, USA), respectively. For this procedure, culture media were collected at predetermined time points (7, 10, 14 and 21 d) and stored at +4 °C until analysis. Values were normalized with cell-free samples.
2.17. Statistical analysis
All experiments were performed in triplicate, and values are expressed as mean ± standard deviation. Comparisons between groups were made with one- and two-way ANOVA followed by Bonferroni's post-hoc tests. Values are statistically significant at *p < 0.05, **p < 0.01, ***p < 0.001.
3. Results and discussion
PRP gel (or fibrin gel) is usually obtained by mixing liquid PRP with exogenous activators such as thrombin, calcium salts, ascorbic acid, batroxobin or collagen [28]. The main limitation of PRP gels is that they do not have sufficient mechanical strength and stability [29]. To overcome this limitation, using PRP or PL directly in the fabrication of tissue scaffolds could be a new strategy. Pan et al developed a cryogel scaffold by cross-linking PRP proteins with alginate using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and N-hydroxysuccinimide for articular cartilage defect repair [30]. In another study, an intrinsically bioactive cryogel based on PL and aldehyde-functionalized cellulose nanocrystals (a-CNCs) was developed for hemostasis applications [31]. We were the first to develop a PRP cryogel (PL/OD) scaffold without the need for any chemical initiator and evaluated the proliferation and in vitro chondrogenic differentiation of hASCs cultured on this construct [19]. The resulting PRP cryogel scaffold was found to have an interconnected macroporous structure that allows for high elasticity, cell migration, and physiological fluid transfer. These promising findings led us to examine the adaptation of the platform to bone tissue engineering. The presence of chemically modified dextran in the composition of the PRP scaffold can contribute to the similarity of the ECM and may also improve cell adhesion properties.
3.1. SEM findings
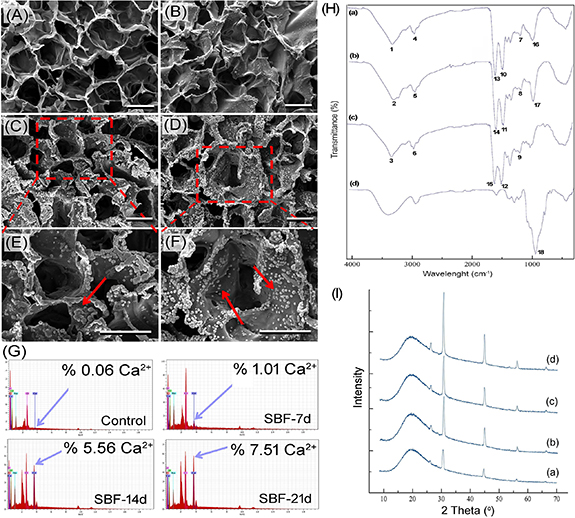
SEM was used to evaluate the morphology of the resulting composite scaffold and to observe the formation of mineral deposition on the scaffold surfaces after SBF incubation (figures 2(A)–(F)). SEM images showed that the mineralized PL/OD scaffold has a porous structure and SBF incubation produced apatite nucleation on the pore walls and surfaces of the construct [32]. The process of biomineralization on the scaffold surface was successfully achieved by the formation of an apatite layer. It is also clearly seen that there is an increase in the amount of apatite nucleation depending on the SBF incubation time [33]. EDS spectra from a random location in the scaffold surfaces were performed to further verify the presence of apatite deposition on the PL/OD scaffold surfaces. EDS spectra (figure 2(G)) showed successful precipitation of calcium and the increase in calcium element in the PL/OD scaffolds during the mineralization process [34]. The results indicated that the mineralization of the PL/OD scaffolds was successfully achieved, and the calcium ion level increased with increasing SBF incubation time [35].
Figure 2. SEM images of PL/OD scaffolds incubated in SBF for 0 d (A), 7 d (B), 14 d (C), (E), and 21 d (D), (F). Scale bars = 50 µm. (G) EDS spectra of the PL/OD scaffolds and calcium amounts released from the hOBs cultured on the mineralized and non-mineralized scaffolds. (H) FTIR spectra of non-mineralized (a), mineralized scaffold (b), PL (c) and OD (d). (I) XRD patterns of PL/OD scaffolds incubated in SBF for 0 d (a), 7 d (b), 14 d (c), and 21 d (d).
Download figure:
Standard image High-resolution image3.2. FTIR analysis
FTIR spectroscopy was applied to evaluate the functional groups present in PL, OD and PL/OD scaffolds based on the peak values in the IR. FTIR diagrams of non-mineralized and mineralized PL/OD scaffolds, PL and OD are shown in figure 2(H). The peaks at 3265–3280 cm−1 (1, 2 and 3) are attributed to the O-H and free amine groups. The peaks at 2926–2928 cm−1 (4, 5 and 6) are related to C-H weak vibration. Peaks at 1239–1241 cm−1 indicate amide III, CN stretching, NH bending (7, 8 and 9). The peaks at 1516–1531 cm−1 are attributed to amide II, CN stretching, NH bending (10, 11 and 12), and the peaks at 1634–1635 cm−1 (13, 14 and 15) show the amide I and C=O stretching [36, 37]. In addition, the peaks observed at 1039–1043 cm−1 (16 and 17) were associated with the phosphate group (PO4 3−) [38]. There was an increase in the intensity of the peaks at 1039–1043 cm−1 compared to the non-mineralized constructs, confirming the formation of mineral deposited on the scaffolds after incubation of the PL/OD scaffolds in SBF. The peak observed at 1040 cm−1 (18) was characteristic of C–O stretching on the polysaccharide backbone [39].
3.3. XRD analysis
Crystal phase identification of PL/OD scaffolds was performed by XRD analysis. Figure 2(I) shows the XRD patterns of non-mineralized (a) and mineralized (b)–(d) PL/OD scaffolds. The broad peak observed around 20° was associated with amorphous OD [40, 41]. Crystal diffraction peaks were observed at 2θ = 31.5°, 45.2°, 56.2° and 66° in all XRD diffractograms. Especially the diffraction peak observed at 31.5° may be related to chloroapatite  Ca10Cl2(PO4)6] minerals (JCPDS card No. 00–001-1011, supplementary figure 1) [42, 43]. On the other hand, other diffraction peaks observed at 45.2°, 56.2°; and 66° may have originated from NaCl crystallization in the scaffolds [44]. Interestingly, the non-mineralized PL/OL scaffold also had the same diffraction patterns. This is probably the result of the ionic composition of the PRP samples used. Ions existing in PL can form apatite and NaCl minerals on non-mineralized PL/OD scaffolds after lyophilization of the molded PL/OD cryogel. This can be supported by the SEM-EDS findings. As seen in figure 2(G), significant amounts of Ca, Na, P, and Cl were present in the non-mineralized scaffold, which could form apatite and NaCl minerals. These minerals acted as seeds, and the SBF solution promoted the growth of crystals on the scaffolds. As a result, the intensity of the diffraction peaks related to chloroapatite and NaCl increased in parallel with the prolongation of the mineralization time [35]. As seen in figure 2(I), they reached their maximum at day 21.
Ca10Cl2(PO4)6] minerals (JCPDS card No. 00–001-1011, supplementary figure 1) [42, 43]. On the other hand, other diffraction peaks observed at 45.2°, 56.2°; and 66° may have originated from NaCl crystallization in the scaffolds [44]. Interestingly, the non-mineralized PL/OL scaffold also had the same diffraction patterns. This is probably the result of the ionic composition of the PRP samples used. Ions existing in PL can form apatite and NaCl minerals on non-mineralized PL/OD scaffolds after lyophilization of the molded PL/OD cryogel. This can be supported by the SEM-EDS findings. As seen in figure 2(G), significant amounts of Ca, Na, P, and Cl were present in the non-mineralized scaffold, which could form apatite and NaCl minerals. These minerals acted as seeds, and the SBF solution promoted the growth of crystals on the scaffolds. As a result, the intensity of the diffraction peaks related to chloroapatite and NaCl increased in parallel with the prolongation of the mineralization time [35]. As seen in figure 2(I), they reached their maximum at day 21.
3.4. Porosity measurements
Mercury porosimetry curves are presented in figure 3(A). The pore size distribution results show that all scaffolds have a wide pore size distribution ranging from 10 µm to 200 µm. In the case of in vivo transplantation, it may be possible that the macropores in the scaffold provide space for vascularization and innervation, and the micropores support capillary infiltration during bone growth. Structures with pore size distributions ranging from a few micrometers to several hundred micrometers are considered to be scaffold candidates suitable for bone repair [45]. Our findings indicate that PL/OD scaffolds with a wide pore size distribution may be suitable in this respect for autologous bone tissue engineering.
Figure 3. Pore size distribution from mercury porosimetry curves (A), theoretical porosity ratios (B), water uptake capacities (C), elastic modulus (D), TGA curves (E) and DTG curves (F) of the PL/OD scaffolds incubated in SBF for different times. Values are statistically significant at * p < 0.05, ** p < 0.01, *** p < 0.001.
Download figure:
Standard image High-resolution imageDensities of mineralized and non-mineralized PL/OD scaffolds were determined based on Archimedes' principle. The theoretical porosity ratios were calculated by the liquid displacement method using ethyl alcohol. Calculations revealed that all PL/OD scaffolds have a porosity percentage ranging from 51.5% to 57%, consistent with other studies of bone repair [46]. The resulting increase in chloroapatite deposition on the scaffolds with prolongation of the SBF incubation time resulted in an insignificant decrease in the percentage of porosity from 57% to 51.5% (figure 3(B)).
3.5. Water retention capacity
The water retention capacities of the scaffolds are presented in figure 3(C). In general, all cryogels showed high water retention as expected. The water uptake percentages of the cryogel scaffolds incubated for 0, 7, 14 and 21 d in SBF were measured as 1300 ± 19%, 1242 ± 13.6%, 1171 ± 13.7% and 1183 ± 35.8%, respectively. With the prolongation of the SBF incubation period, a slight decrease (at 7 d and 14 d) was observed in the water retention capacity of the PL/OD scaffolds due to mineral accumulations on the surfaces. In addition, it was determined that mineralized PL/OD scaffolds incubated in SBF for 14 and 21 d had an almost constant and high water absorption capacity. As is known, PRP-based bioactive cryogel scaffolds act as a carrier or reservoir for growth-promoting signals in enhancing tissue regeneration. It is possible that bone repair potential is enhanced by incorporating osteogenic mineral deposits into the bioactive cryogel scaffold.
3.6. Mechanical test
Compression tests were carried out to examine the mechanical properties of the prepared scaffolds after SBF incubation. The elastic modulus values obtained from the compression tests are given in figure 3(D). As a result, while the elastic modulus was 0.061 N mm−2 in the control group, this value was measured as 0.072 N mm−2, 0.081 N mm−2 and 0.11 N mm−2 after 7, 14 and 21 d of incubation in SBF, respectively. Here, it is seen that the elastic modulus of the scaffolds increases as the mineralization time increases. The results showed that SBF incubation caused an increase in the elastic modulus of the scaffolds. This indicated that SBF incubation led to increased mechanical strength and stiffness [47] due to the formation of a chloroapatite layer on the scaffold surface compared to non-mineralized scaffold.
3.7. Thermal analysis
TGA was performed to determine the effect of SBF mineralization on the thermal properties of PL/OD scaffolds. As can be seen in figure 3(E), the mass loss of the scaffolds decreased as the mineralization time increased. Final weights remaining at 650 °C were determined as 25.3%, 27%, 27.2% and 28.6% for biomineralized PL/OD scaffolds at 0, 7, 14 and 21 d, respectively. Here, it is clearly observed that PL/OD scaffolds with the 7 and 14 d SBF incubation times showed similar weight losses. On the other hand, the peaks in the DTG curves (figure 3(F)) indicated that the maximum degradation rate temperature was around 327 °C for non-mineralized scaffolds, while this value was 340 °C for mineralized scaffolds. The peaks of the mineralized scaffolds shifted to higher temperature compared to non-mineralized scaffolds, suggesting the higher thermal stability of the mineralized scaffolds than that of the non-mineralized scaffold [48].
3.8. In vitro cytotoxicity
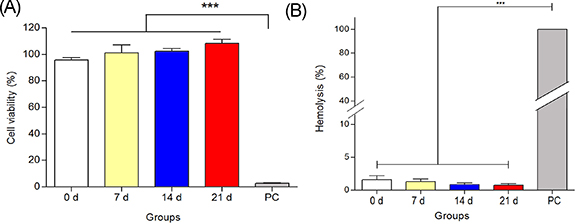
In vitro cytotoxicity test was performed according to the ISO 10993-5 standard to evaluate the in vitro biocompatibility of PL/OD scaffolds (figure 4(A)). The results showed that the viability of cells cultured with scaffold extract incubated in SBF for 7 d was 100.5%. In 14 and 21 d of SBF incubation, cell viability levels were determined as 102.2% and 108.2%, respectively. Cell viability was found to be 94.1% for the non-mineralized PL/OD scaffold. The findings clearly show that there is no significant difference between mineralized and non-mineralized PL/OD scaffolds. In the PC, the viability of the cells was very low, at the level of 0.39%, as expected. These findings are in line with previous studies reporting that PL/OD scaffolds are biocompatible [19]. It is demonstrated in this study that mineralization process did not adversely affect biocompatibility; mineralized PL/OD scaffolds did not show any toxic effects and supported the viability and proliferation of hOBs. This study reveals that the biocompatibility of PL/OD scaffolds is not affected as a result of mineralization and that mineralized PL/OD scaffolds support the in vitro viability and proliferation of hOBs without any signs of toxic effects.
Figure 4. In vitro cytotoxicity (A) and hemolysis results (B) of the PL/OD scaffolds incubated in SBF for different times. Values are statistically significant at *** p < 0.001. All groups were compared with positive control (PC) using one-way ANOVA followed by Bonferroni's post-hoc test.
Download figure:
Standard image High-resolution image3.9. In vitro hemolysis
To determine the hemocompatibility of the PL/OD scaffolds, in vitro hemolysis testing was performed on the samples according to the ISO 10993-4 standard. The results showed that the scaffolds incubated in SBF for 7 d showed a hemolysis value of 1.13%, while the scaffolds incubated in SBF for 14 d and 21 d had hemolysis values of 0.75% and 0.62%, respectively (figure 4(B)). The findings revealed that all measured levels of hemolysis were well below the accepted upper limit for hemolysis in biomaterials, thus the developed biomineralized scaffolds were considered to have no hemolytic effect [49].
3.10. SEM and EDS analyses
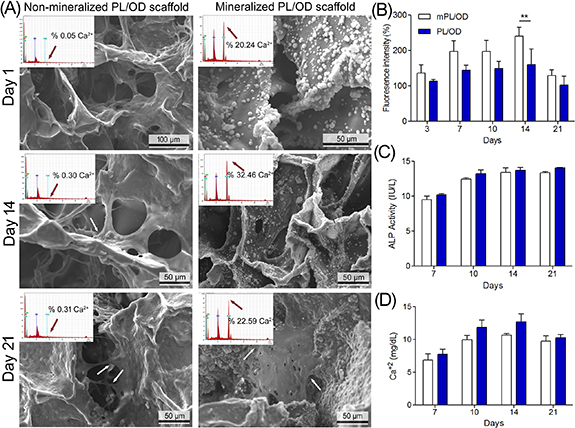
SEM analysis was used to morphologically evaluate the interaction of cells with the PL/OD scaffolds and to observe the amount of calcium deposited in the cell seed scaffolds. SEM images clearly show that cells adhered and spread on the scaffold surface (figure 5(A)).
Figure 5. SEM images and EDS spectra of the non-mineralized and mineralized PL/OD scaffolds cultured with hOBs for different times (A). Arrows indicate numerous filopodia extending from the hOBs after 14 and 21 d. AlamarblueTM results are expressed as a percent of first day (B). ALP (C) and calcium (D) amounts released from the hOBs cultured on the mineralized and non-mineralized scaffolds after 7, 10, 14, and 21 d. Statistical analysis was performed using two-way ANOVA. Values are statistically significant at ** p < 0.01.
Download figure:
Standard image High-resolution imageEDS analysis was conducted to evaluate mineralization and calcium deposits in the ECM of hOBs seeded on non-mineralized and mineralized PL/OD scaffolds for 1, 7 and 21 d. Quantitative elemental EDS analysis of PL/OD scaffolds confirmed the presence of the calcium element. After biomineralization, an increase in the amount of calcium in the scaffolds was observed as expected [50, 51]. The results showed that the amount of calcium deposition on mineralized PL/OD scaffolds increased up to day 14 of culture, and then decreased by day 21; this finding is consistent with the calcium release results (figure 5(D)).
3.11. alamarBlueTM analysis
Investigating the viability of cells seeded on a scaffold is important in determining the suitability of that biomaterial for future tissue engineering applications [52, 53]. For this, possible changes in the metabolic activity of cells seeded on mineralized and non-mineralized PL/OD scaffolds were examined by alamarBlue™ analysis for up to 21 d and the results are shown in figure 5(B). In cell culture experiments, non-mineralized PL/OD scaffolds were compared with PL/OD scaffolds incubated in SBF for 14 d. The results generally revealed that the fluorescence intensities of cells growing on both scaffold surfaces increased over time, peaking at day 14 and decreasing slightly at day 21 of culture. Secondly, the mineralized PL/OD scaffolds showed a higher metabolic activity compared to non-mineralized scaffolds. This finding can be explained by the fact that mineral accumulation on the scaffolds increases the surface area and therefore leads to increased proliferation of osteoblastic cells [54]. At day 14 of culture, a significant difference in metabolic activity was observed between mineralized and non-mineralized scaffolds (** p < 0.01) (figure 5(B)). alamarBlueTM findings clearly indicated that the cells could easily attach, proliferate and maintain their viability throughout the culture period on the PL/OD scaffolds.
3.12. ALP and calcium release
Release of ALP and calcium from hOBs cultured on mineralized and non-mineralized scaffolds after 7, 10, 14 and 21 d periods were determined using ALP and Calcium assay kits. As can be seen in figure 5(C), ALP activity of hOBs growing on both types of scaffolds increased over time. There was no significant difference between the ALP activities of cells cultured on mineralized and non-mineralized scaffolds. The fact that mineral deposition on the scaffold did not increase ALP activity in hOB cells may be related to the accumulation of calcium ions in extracellular vesicles [55]. On the other hand, calcium release from the cells grown on both types of scaffolds increased over time and reached its highest value at day 14, and slightly decreased on the 21st day (figure 5(D)). The difference in calcium release levels from cells cultured in mineralized and non-mineralized scaffolds was not significant, but EDS results showed higher calcium contents in the mineralized group. This can be explained by the fact that the EDS analysis provides information on only a part of the scaffold, not the whole. The results show that mineralized PL/OD scaffolds serve as a suitable substrate that allows hOBs to retain their properties in vitro. However, the in vivo properties of mineralized PL/OD scaffolds will need to be investigated in the future.
4. Conclusion
Despite the availability of a large number of approaches, large bone defects cannot be fully regenerated due to the limitations of bone defect repair per se. Therefore, there is a need to develop bioactive composite scaffolds with a biomineralized matrix structure that has the potential to accelerate bone regeneration. Until now, in many studies carried out in the area of tissue engineering, PRP has been used as a biomaterial additive for a function to increase the wound healing effect of biomaterials. PRP-based biomaterials with temporary support in tissue engineering applications are thought to have great potential for regenerative therapies due to their growth factor content. In this study, biomineralized PRP/OD-based macroporous cryogels were developed and characterized for potential application in bone tissue engineering, as a xeno-free (and potentially autogenic) bioactive 3D scaffold for future bone repair applications. However, further studies will be needed to evaluate the safety, osteogenic potential, and bone healing effectiveness of the scaffolds in vivo.
Data availability statement
Data will be made provided upon reasonable request.
The data that support the findings of this study are available upon reasonable request from the authors.
CRediT authorship contribution statement
Şükran Şeker: Conceptualization, Methodology, Investigation, Data curation, Writing. Dilara Aral: Conceptualization, Investigation, Data curation, Formal analysis, Writing. Ayşe Eser Elçin: Data curation, Writing. Yaşar Murat Elçin: Supervising, Resources, Conceptualization, Methodology, Writing—reviewing & editing.
Conflict of interest
Y M E is the founder and shareholder of Biovalda Health Technologies, Inc. (Ankara, Turkey). Ş Ş, A E E and Y M E have patent applications in relation to regenerative biomaterials. The authors declare no competing financial interests in relation to this particular article. The authors are alone responsible for the content and writing of the paper.
Supplementary data (0.3 MB PDF)