Abstract
Synthetic orthopaedic materials consisting of a single bioinert polymeric material do not meet the complex biological and physical requirements of scaffold-guided bone tissue repair and regeneration. Of particular interest is the design of biocompatible hydrogel-hydroxyapatite composite bone substitutes with outstanding interfacial adhesion that would warranty the ability for the composite to withstand functional loadings without exhibiting brittle fractures during the dynamic guided tissue regeneration. For this purpose, the hydroxylated side chain of chemically cross-linked poly (2-hydroxyethyl methacrylate) (pHEMA) is substitute with a carboxylated side chain to make poly (glycerol methacrylate) (pGLYMA). Here, we carry out atomistic simulations and atomic force microscopy to predict and experimentally determine the interfacial adhesion energies of pHEMA and pGLYMA with the surface of single-crystalline hydroxyapatite (HA) whiskers. Both experimental and numerical results showed that pGLYMA has stronger adhesion forces with HA and may be used for preparing a high-affinity polymer-HA composite. The high adhesive interactions between pGLYMA and HA were found to be due to strong electrostatic energies.
Export citation and abstract BibTeX RIS
1. Introduction and background
Design of biomimetic synthetic scaffolds for guided repair and regeneration of skeletal tissues are of significant clinical interest [1]. Properly designed bone tissue engineering scaffolds hold the promise to overcome the limitations of autogenic bone grafts (donor site morbidity and limited quantity) and allogenic bone grafts (high failure rate and risk for infections) for the repair of critical-size bone defects [2, 3]. Natural bone is a composite of collagen, a protein-based hydrogel template, and inorganic dahilite crystals (with hydroxyapatite (HA) being the major inorganic component). The unusual combination of a hard inorganic material and an underlying elastic hydrogel network endows native bone with unique mechanical properties, such as resistance to tensile and compressive forces and high fracture toughness [4, 5]. Photocrosslinked polymethacrylates and their functionalized copolymers, characterized with their high water- retention capacity and biocompatibility, are attractive hydrogels for tissue engineering applications. One major challenge for their application as a three-dimensional (3D) synthetic bone substitute, however, is to realize high-affinity integration of HA with the hydrogel scaffold [6, 7]. Song et al previously showed that poly (2-hydroxyethyl methacrylate), or pHEMA, a well-studied biocompatible synthetic hydrogel polymer, could be surface mineralized with nanocrystalline HA with robust interfacial adhesion using a novel heterogeneous mineralization method [8–10]. Furthermore, Song et al demonstrated that up to 50 wt% HA could also be structurally integrated with the 3D interior of the pHEMA hydrogel to generate structural composites that can resist high compressive loads without exhibiting brittle fractures [11]. The side chains of pHEMA are terminated with hydroxylated residues, which are richly present in type I collagen [12] and are believed to be responsible for the observed favourable integration of pHEMA with HA. These structural composites have also shown great in vivo performances for scaffold-guided repair of critical long-bone defects in rodents [13, 14]. Here we hypothesize that the pHEMA derivatives, when functionalized with other appropriate mineral- nucleating side chains [15], may further improve the interfacial adhesion strength in these structural composites. In particular, we hypothesize that the negatively charged carboxylate residue, a common motif found in acidic non-collagenous bone matrix proteins and recognized to play important roles in mediating templated biomineralization [16–18], would be a rational biomimetic element to be incorporated in the side chain of functional pHEMA. To test this hypothesis, a reliable method to quantitatively evaluate the adhesion energies between potential HA-binding ligands and HA crystals on an atomic scale needs to be developed. In this paper, we investigated the interfacial affinity between well-defined surfaces of HA single-crystal whiskers [19] and pHEMA versus poly (glycerol methacrylate) (pGLYMA), which are functionalized with hydroxylated and carboxylated side chains, respectively. A combination of atomistic simulations and atomic force microscopy (AFM) experiments was used to study the pHEMA and pGLYMA pairwise adhesion energies with a well-defined HA crystal. Models of cross-linked structures of pHEMA and pGLYMA were used to simulate the elastic properties of the two polymers and to predict the adhesion energies at the interfaces of pHEMA/HA and pGLYMA/HA. The simulation results were then verified by a novel AFM experimental method developed earlier by Rahbar et al [20, 21]. The details of the experimental method and computational process are presented in the following sections.
2. The experimental measurement of adhesion
AFM was used to measure the adhesion forces between the HA single-crystal whisker surface (100) and the two polymers, pHEMA and pGLYMA. The HA whiskers were prepared by molten salt synthesis [14]. The AFM tips were coated with pHEMA and pGLYMA by a multi-step coating process and validated by scanning electron microscopy (SEM).
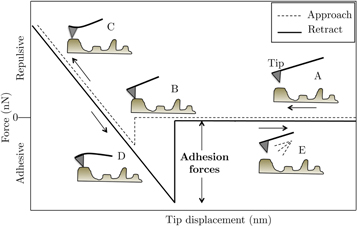
The AFM-coated tips, in contact mode, were brought into contact with the coupons of HA whiskers, with the (100) faces exposed, and pulled off. The cantilevered tip begins at point A, where there is no deflection of the tip (figure 1). As the tip approaches the surface, adhesive forces between the tips and the substrate pull down the tip onto the surface (point B). Subsequently, the tip bends under elastic deformation as the deflection increases up to point C. The tip then is retracted from the surface. However, the adhesive forces prevent detachment of the tip and cause a bending deflection (point D). The reversed loading must be continued to point E, at which the force is sufficient to overcome the adhesive interactions. The maximum deflection is recorded for the force calculations by using the equation of a cantilever beam that correlates the deflections with the forces.
Figure 1. Schematic of the interaction of AFM tips with surfaces in the contact mode.
Download figure:
Standard image High-resolution image2.1. Sample preparations
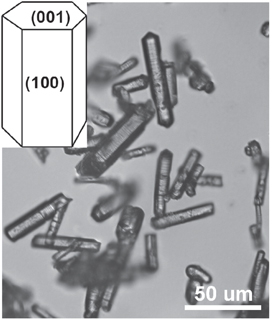
HA single-crystal whiskers were prepared using molten salt synthesis [19]. Briefly, commercial polycrystalline HA powders were dry-mixed with potassium sulfate at a K2SO4/HA weight ratio of 1.6. The mixture was placed in a clean crucible and heated in a high-temperature furnace from room temperature to 1190 °C at a rate of 5 °C min−1 and held at 1190 °C for 3.5 h before it was cooled to room temperature. The single-crystal HA whiskers were separated from the solid mass by washing with Milli-Q water at 90 °C three times. The whiskers were then air-dried and characterized by optical microscopy to reveal morphologies (figure 2) consistent with the literature [19].
Figure 2. Optical micrograph of the single-crystal HA whiskers prepared by molten salt synthesis and the schematic (inset) of the (100) face to be utilized for the AFM study.
Download figure:
Standard image High-resolution imageFunctional monomer GLYMA was prepared and purified, as previously reported [15]. Commercial HEMA (Aldrich) was freshly distilled under reduced pressure, and EGDMA (Aldrich) was passed through resins to remove radical inhibitors prior to use.
To generate a robust layer of surface coating of pHEMA or pGLYMA on the AFM tip, we first covalently bonded a layer of thiolated methacrylate on gold-coated AFM tips through the high-affinity Au-S interaction. The methacrylate end group exposed to the surface is designed to not only improve the wetting of the hydrogel cocktail (HEMA/EGDMA or GLYMA/EGDMA along with radical initiators) with the AFM tip, but also to participate in the radical crosslinking with the respective monomer and crosslinker to generate a covalently bound pHEMA and pGLYMA surface coating. Specifically, the AFM tips were sputter-coated with 10 nm chromium-nickel and afterwards a layer of 30 nm gold [22]. The AFM tips were then immersed in ultrapure ethanol, which contained 0.5 mM 2-(methylthio) ethyl methacrylate, at room temperature for 24 h, washed several times with ultrapure ethanol afterwards, and dried under nitrogen. These tips were then dipped into, respectively, freshly prepared cocktails of hydrogel monomer (100 mg HEMA or GLYMA) and crosslinker (2 μL EGDMA) in a mixture of MilliQ water (20 μL), ethylene glycol (30 μL), and aqueous solution initiators sodium metabissulfite (150 mg mL−1, 10 μL) and ammonium persulfate (400 mg mL−1, 10 μL), following ratios previously published [15], and crosslinked under ultraviolet (254 nm) for 1 min As shown in figure 3, this strategy successfully led to the uniform coating of the AFM tips with a thin layer of pHEMA or pGLYMA crosslinked by 2 wt% EGDMA.
Figure 3. SEM micrographs of the AFM tips before and after coating with a thin layer of pHEMA or pGLYMA crosslinked by 2 wt% EGDMA. The 'defective' hydrogel at the base of the coated tips was caused by beam damage during the imaging.
Download figure:
Standard image High-resolution imageIn order to acquire a reliable AFM measurement, even distribution of a single layer of HA whiskers is needed to guarantee that the coated tips will touch the HA whiskers rather than the underneath surface. The HA whiskers also need to be stably fixated to the substrate to prevent their movements during the retraction phase. Accordingly, a thermal wax was used to mount the HA whiskers on the glass substrate [23]. Specifically, a piece of wax was placed on the glass substrate and heated up to 70 °C until the wax softened. Immediately upon removing the glass substrate from the heater, the HA whiskers were sprinkled over as the wax started to solidify. After 15 min at room temperature, the thermal wax becomes solid. The surface of the substrate was brushed carefully to remove large agglomerates of the HA, leaving a layer of scattered HA whiskers, predominantly exposing their (100) faces.
In order to merely measure the adhesion forces between the hydrogel-coated AFM tips and the HA whiskers, two samples were prepared: one with only paraffin wax and one with HA whiskers sprinkled over and adhered to the surface of the wax. Measuring the adhesion of the AFM tips with the wax-only control helps us determine whether the tip was in contact with the HA whiskers or with the wax during the measurement. Multiple points of the samples were tested to get the adhesion forces between the tips and HA crystals. For each point 10 adhesion-force measurements were collected for the proper statistical analysis.
2.2. AFM experiments and data analysis
The AFM tips were brought in contact with HA whiskers in the contact mode of AFM. The adhesion forces were computed from the maximum deflection of the tips times a constant, which is the stiffness of the beam. The deflections were obtained from the difference between point E and A of the approaching-retracting curve in figure 1.
To calculate the adhesion energies from the measured forces, different contact theories were developed by Maugis–Dugdale (MD) [24], Derjaguin–Muller–Toporov (DMT) [25], and Johnson–Kendall–Roberts (JKR) [26]. The JKR model considers the contact as compliant with the large radius of curvature. The DMT is suitable for stiff contacts with a small radius of curvature. The MD model is an intermediate case between JKR and DMT that decides which contact condition is applicable for modeling the adhesion between two surfaces by defining a transition parameter λ,
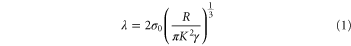
where σ0 is the maximum attraction stress. R is the combined reduced radius of the two surfaces in contact (AFM tips and HA particles), which can be calculated from Rrms, the root-mean-squared roughness of the substrate and Rtip, the radius of the coated AFM tip given by,
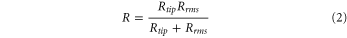
and K is the reduced modulus of elasticity of the tip and sample given by,
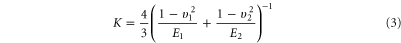
where E1 and E2 are the Young's moduli and υ1 and υ2 are Poisson's ratios of the coated AFM tips and the HA crystals, respectively.
For values of λ < 0.1, the DMT model is valid, while the JKR theory is the relevant model for values of λ > 5.0. Consequently, values of λ between 0.1–5 correspond to the intermediate situation. In this study MD equations were used to calculate the λs and the adhesion energies (ϒ) between the polymers and the HA particles. Youssefian et al discussed the calculation process of adhesion energies from MD equations in detail [21].
3. Atomistic simulations of interaction between pGLYMA and pHEMA with HA crystals
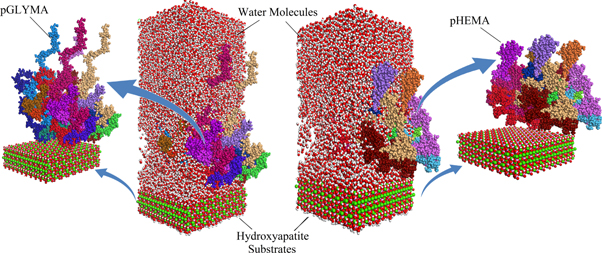
Molecular dynamics techniques were used to simulate the adhesion between the polymers and HA particles. The COMPASS (condensed-phase optimized molecular potentials for atomistic simulation studies [27]) force field, a suitable force field for atomistic simulation of condensed matters, was used to simulate the physical and mechanical properties of the polymers. It is one of the first ab initio-based force fields that is parameterized and validated with the experiment results using condensed-phase properties [27]. Therefore, it is an accurate and reliable force field for predicting mechanical, structural, and thermodynamic properties of a vast range of molecules and atoms. Nevertheless, the performance of this force field in atomistic simulations of the HA, pHEMA, and pGYMA was verified in this study.
3.1. Modeling hydroxyapatite surface
In order to model the HA surface, hexagonal unit cells with a symmetry group of P63 and lengths of a = b = 9.421 Å, c = 6.881 Å, and the angles of α = β = 90° and γ = 120° were modeled and optimized using the steepest-descent approach, followed by the conjugate guardian method. The non-bonded summations were calculated using Ewald for electrostatic, with an accuracy of 0.001 kcal mol−1, and atom-based for van der Waals, with truncation of atoms further than the cutoff distance of 12.5 Å. The properties of the model were obtained to assess the suitability of the COMPASS force field for atomistic modeling of HA. The computed density of HA from atomistic simulation was about 3.10 g/cc, which was close to the experimental value of 3.14 g/cc The modulus of elasticity was estimated to be 40.30 GPa, 40.30 GPa, and 62.15 GPa in the X, Y, and Z directions, respectively. The experimental values for these properties depend on the volume fraction and porosities and vary between 7–103 GPa [28–31]. The Poisson's ratio of HA is also a function of volume fraction and porosities and changes from 0.15 to 0.3. The estimated Poisson ratio from atomistic simulation was 0.29.
The HA unit cell was cleaved on the (100) surface, which is the predominant surface of the HA whiskers exposed to AFM tips in the AFM experiments. The (0 1 0) surface was also created to be used in the simulations, because this plane is the major plane in the morphology of the biological material due to the effect of the collagen matrix [32]. Furthermore, the electrostatic characteristic of this plane is not neutral due to a negatively charged OH group on one surface and positively charged Ca atoms on the other surface. A super cell with the dimensions of 3.44 × 3.77 × 1.57 nm3 was created from the equilibrated (0 1 0) and (1 0 0) HA surfaces.
3.2. Modeling of the polymers
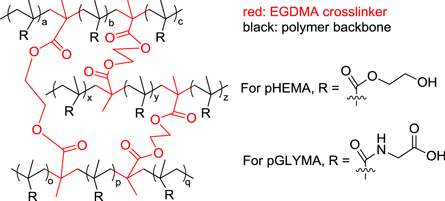
Crosslinking a polymer is widely believed to improve the mechanical properties. Here, ethylene glycol dimethacrylate (EGDMA) was used as the crosslinking agent for pHEMA and pGLYMA. These polymers were crosslinked with 2% (by weight) EGDMA, according to the standard radical polymerization protocol [8, 33]. Therefore, the molecular mass ratio of the crosslink agent (EGDMA) to the hydrogel monomers was found to be 0.0129. The number of repeating units of EDGM, e, HEMA, h, and GLYMA, g, can be calculated by the following equation:

By choosing three EDGMA units, we need 234 HEMA or GLYMA to have a correct ratio of the polymers to the crosslinked agent. These 234 repeating units should create three chains of the polymer that are crosslinked by the three agents. Hence, there should be 80 repeating units in each polymer chain: 78 repeating units  plus two units as the connection site for the agents (figure 4).
plus two units as the connection site for the agents (figure 4).
Figure 4. Depiction of the crosslinked hydrogel molecular networks.
Download figure:
Standard image High-resolution imageThree oligomers of polymer with 80 repeating units along with three molecules of EDGMA were constructed in periodic boundaries with the dimensions of 3.2 × 3.2 × 1.7 nm3. Each cell was filled with 3942 water molecules to simulate the real conditions. The cell was going through a relaxation process [34] that starts with compressing at high pressure (5000 bar) for 200 ps at 300 K, followed by NVT dynamics at 600 K and 300 K, successively, for 20 ps. Subsequently, an NPT dynamics was performed at 1 bar and 300 K for 200 ps. If the density was lower than the experimental density, the first two steps were repeated. The final simulation cells had dimensions of 3.4 × 3.4 × 3.4 nm3 with a density of 1.2 g/cc and 1.27 g/cc for pHEMA and pGLYMA, respectively. These values are in good agreement with the experimental values.
To compare the mechanical properties of pHEMA and pGLYMA, the Young's moduli of the polymers were calculated. The periodic structures of polymers were expanded along each direction to the maximum strain amplitude of 0.04 in nine steps. In each step the stresses were obtained from virial stress expression:
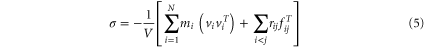
which is commonly used to relate the computed stress in molecular dynamics to continuum stresses.
To simulate the interaction of the interfaces of the polymers and HA, multiple assemblies of polymer chains on top of the HA substrate were created. Three different configurations of each polymer were chosen to remove the effect of the initial conditions on the results. After optimizing the polymers, NVT dynamic simulations were performed at 300 K for 300 ps with time steps of 1 fs. The interaction energy was calculated from the final trajectories of simulations, by using the following equation:
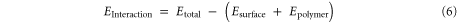
where Etotal is the energy of the HA surface and the polymer, Esurface is the energy of the HA surface without the polymer, and Epolymer is the energy of the polymer without the HA surface.
4. Results and discussion
4.1. Experimental measurement of adhesion energies
In order to ensure that the tips merely touch the HA whiskers, first they were tested on the smooth surface of paraffin wax with an average surface roughness of ∼ 97 nm, as shown in figure 5. The tips, then, were tested on the surface of paraffin with the HA whiskers (figure 6), which has a roughness in the micron range. In order to ensure that the measured adhesion is on the (100) surface of the HA whiskers, AFM taping mode was used to take an image of the surface. The static mode was used, then, to obtain multiple force curves of the desired points.
Figure 5. A typical 3D image of the surface of the thermal wax samples. The smooth surface of the wax was used to detect the HA particles on the substrate.
Download figure:
Standard image High-resolution imageFigure 6. A single layer of HA particles fixed firmly in the thermal wax. This method helps the alignment of the HA crystals in the desired direction.
Download figure:
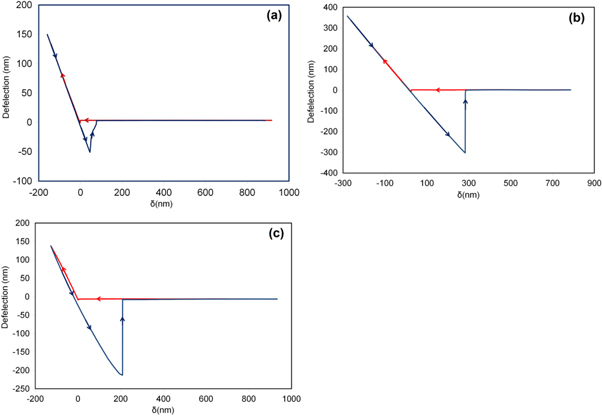
Standard image High-resolution imageThe adhesion forces between the tips and the paraffin were computed from the deflection-displacement curve of the tips and paraffin wax (figure 7(a)) to be about 23.72 nN. Figure 7(b) and (c) show the deflection-displacement curves of the pHEMA- and pGlyMA-coated tips with HA whiskers, which are about 104.4 ± 18 nN and 152.5 ± 22 nN, respectively. The statistical analysis shows higher adhesion forces between the HA whiskers and the AFM tips coated with pGLYMA and pHEMA than the adhesion forces between the paraffin wax and the tips. This implies that the tips only measured the adhesion between the HA and hydrogels.
Figure 7. (a) Typical deflection-separation curve of AFM tips coated with pHEMA tapping on paraffin. Typical deflection-separation curve of AFM tips coated with (b) pGLYMA and (c) pHEMA tapping on the HA surface. The stiffness of the tips is very close for different polymers, and the analysis shows a higher adhesion force between pGLYMA/HA than the pHEMA/HA particles.
Download figure:
Standard image High-resolution imageTo find the adhesion energies per unit area from the MD model equations, h0 was considered to be half of the non-bonded cutoff distance, which is 12.5 Å. Since the width of the HA whiskers are in the micron meter range, the reduced radius of the adhesion energy is simply the radius of the AFM tips. The radius of the coated AFM tips was measured to be around 175 nm using SEM.
By carrying out iterative calculations, the adhesion energies between the HA whiskers and pGLYMA and pHEMA were found to be 184.3 mJ m−2 with λ = 8.45 and 126.2 mJ m−2 with λ = 8.41, respectively (table 1). Since λ > 5 corresponds to JKR, the contact between these hydrogels and HA whiskers is compliant with a large radius of curvature. Hence, these results suggest that pGLYMA, with carboxylated side chains, may present a further advantage than the hydroxylated uncharged pHEMA in achieving better bonding affinity with HA in the design of hydrogel-hydroxyapatite structural composites.
Table 1. Experimental results of adhesion forces and energies.
| Tip Stiffness (N m−1) | Force (nN) | Adhesion (mJ m−2) | |
|---|---|---|---|
| pGlyMA | 0.58 ± 0.02 | 152.5 ± 22 | 184.3 ± 20 |
| pHEMA | 0.61 ± 0.01 | 104.4 ± 18 | 126.2 ± 16 |
4.2. Atomistic simulations of adhesion energies
The Young's moduli of the pHEMA and pGLYMA, computed from the stiffness matrices, are presented in table 2. The average Young's moduli of pHEMA and pGLYMA were estimated to be 6.17 ± 0.81 MPa and 9.67 ± 0.55 MPa, respectively. The pHEMA Young's modulus is in good agreement with the respective experimental measurements that are between 1–17 MPa under humidity conditions of 98% and 16% relative humidity, respectively [35]. The results suggest that the pGLYMA exhibits stiffer mechanical behaviour than pHEMA.
Table 2. Young's modulus computed from the simulations.
| Young's Modulus (MPa) | |
|---|---|
| pGlyMA | 9.67 |
| pHEMA | 6.17 |
The adhesion energy per unit area between the hydrogels and different surfaces of HA were calculated from the trajectories of atomistic simulations (figure 8) and shown in figure 9. The adhesion energies between the (100) surface and pGLYMA was estimated at 77.2 mJ m−2, while the adhesion between the same surface and pHEMA was calculated to be around 32.9 mJ m−2.
Figure 8. The results of the molecular dynamics simulations of pGLYMA and pHEMA on the HA (100) surface.
Download figure:
Standard image High-resolution imageFigure 9. The calculated adhesion energy per unit area of pHEMA and pGLYMA on different surfaces of HA.
Download figure:
Standard image High-resolution imageThe adhesion energies between the (010) surface and pGLYMA and pHEMA were estimated to be around 71.9 mJ m−2 and 32.2 mJ m−2, respectively. Comparing simulation results with the experiments suggests that the pHEMA and pGLYMA models can properly estimate the interfacial characteristic between these materials and HA. Although the adhesion energies calculated from the simulation results are less than that of the experiments, the trends are quite consistent. This trend implies that the pGLYMA exhibits a greater adhesive tendency than pHEMA to HA. The components of adhesion energies, electrostatic and van der Waals, were calculated and are presented in tables 3 and 4.
Table 3. The adhesion energy and its components on HA (100).
| Adhesion (mJ m−2) | van der Waals (mJ m−2) | Electrostatic (mJ m−2) | |
|---|---|---|---|
| pGLYMA | 77.26 | 0.52 | 76.74 |
| pHEMA | 54.74 | 2.23 | 52.51 |
Table 4. The adhesion energy and its components on HA (010).
| Adhesion (mJ m−2) | van der Waals (mJ m−2) | Electrostatic (mJ m−2) | |
|---|---|---|---|
| pGLYMA | 74.72 | 1.86 | 72.86 |
| pHEMA | 61.35 | 0.60 | 60.74 |
These results elucidate that the major factor in the interaction between HA and the hydrogel is electrostatic energies, which are higher between HA and pGLYMA than between HA and pHEMA. The main difference between pHEMA and pGLYMA molecular structures is the uncharged side-chain hydroxyl end groups for pHEMA and the negatively charged side-chain carboxylate end groups for pGLYMA. In addition, pHEMA links its side chains to the main chain via an ester linkage, whereas pGLYMA does so via an amide linkage. These two differences cause more electrostatic attraction between the pGLYMA and HA compared to pHEMA and HA ,which results in higher adhesion ennergy.
5. Conclusion
Here we used a combination of atomistic simulations and ATM to predict the mechanical properties of pHEMA and pGLYMA and the interfacial adhesion energies between these hydrogels and the surface of single-crystalline HA whiskers. Both experimental and numerical results showed that pGLYMA has stronger adhesion forces with HA and may be used for preparing high-affinity polymer-HA composites. Further studies showed that the higher interaction between pGLYMA and HA is due to higher electrostatic energies between these materials. This is likely derived from the main difference in molecular structures between pHEMA and pGLYMA, which is the uncharged side-chain hydroxyl end groups for pHEMA and the negatively charged side-chain carboxylate end groups for pGLYMA.
Acknowledgments
This work was supported in part by a UMass Life Sciences Moment Fund.