Abstract
This review paper shows that tungsten should not generally be used as a chronically implanted material.
The metal has a long implant history, from neuroscience, vascular medicine, radiography, orthopaedics, prosthodontics, and various other fields, primarily as a result of its high density, radiopacity, tensile strength, and yield point. However, a crucial material criterion for chronically implanted metals is their long-term resistance to corrosion in body fluids, either by inherently noble metallic surfaces, or by protective passivation layers of metal oxide. The latter is often assumed for elemental tungsten, with references to its 'inertness' and 'stability' common in the literature. This review argues that in the body, metallic tungsten fails this criterion, and will eventually dissolve into the soluble hexavalent form W6+, typically represented by the orthotungstate  (monomeric tungstate) anion. This paper outlines the metal's unfavourable corrosion thermodynamics in the human physiological environment, the chemical pathways to either metallic or metal oxide dissolution, the rate-limiting steps, and the corrosion-accelerating effects of reactive oxidising species that the immune system produces post-implantation.
(monomeric tungstate) anion. This paper outlines the metal's unfavourable corrosion thermodynamics in the human physiological environment, the chemical pathways to either metallic or metal oxide dissolution, the rate-limiting steps, and the corrosion-accelerating effects of reactive oxidising species that the immune system produces post-implantation.
Multiple examples of implant corrosion have been reported, with failure by dissolution to varying extents up to total loss, with associated emission of tungstate ions and elevated blood serum levels measured. The possible toxicity of these corrosion products has also been explored.
As the field of medical implants grows and designers explore novel solutions to medical implant problems, the authors recommend the use of alternative materials.
Export citation and abstract BibTeX RIS
1. Tungsten—an overview
Tungsten (W, atomic number 74), as a result of its unique material characteristics, has long been used to make medical implants. It belongs to the refractory group of metals, those known for their extraordinary resistance to heat and wear [1, 2]. It has the highest melting point of all the metals (3422 °C) [3] and the lowest vapour pressure. It has very high tensile strength [4], and is a hard, tough material that resists buckling forces at small dimensions. It is one of the densest of the naturally occurring elements, at 19.25 g cm−3 (surpassed only by U, Re, Pt, Ir, and Os) [3, 5]. W has the largest cohesive energy of all the elements, including diamond (carbon): 7.9–10.09 eV/atom depending on the theoretical approach [6]. The only stable crystallographic form is α-W, with a body-centred cubic lattice structure [5]. These physical characteristics originate from the nature of tungsten's metallic bonding, with strong unsaturated covalent bonds between the valence 5d orbitals [3]. The interested may pursue tungsten's physical and chemical properties further in reference texts: Lassner and Schubert's [3] work, or Gmelin's Atlas [7].
From a biological perspective, W plays a role only in bio-molecules in niche ecologies, as metal sites bound by protein-derived ligands in tungsto-enzymes in hyperthermophilic archaea [8, 9], usually in cognates to molybdenum [10]. These extremophiles are termed 'obligate anaerobes', and their tungsto-enzymes catalyse redox reactions involving carboxylic group to aldehyde group conversions [3]. There are no other reported instances of W bio-molecules, and no reported functions of W in human biology.
2. Corrosion
The corrosion behaviour of a metal cannot be divorced from the very specific environmental conditions in which it will be expected to operate. It is the interplay of the metal and environment that determines the thermodynamics, kinetics, and pathways of the corrosion process [11]. The operating environment of medical implants can be modelled as wet, chloride-rich saline with reactive highly oxidising species present due to the inflammation response [12, 13]. The pH is typically 7.4, but may vary from 5.6 post-surgery to 9.0 during infection [14, 15]. The effects of various other environmental factors, such as proteins, lipids, and ions, should also be considered.
W has often been referred to as having 'excellent corrosion resistance' [3], and in some biomaterials literature as 'inert' and 'stable' [16, 17] in aqueous solutions [18]. However, in the presence of water, at 25 °C under 1 atm pressure, metallic W is not thermodynamically stable [14, 19, 20]. In neutral aqueous solutions a native WO3 metal oxide film forms but continually dissolves away, and is thus non-passivating [3].
2.1. Corrosion thermodynamics and Pourbaix diagrams
The electrochemical behaviour of W has a complicated pH dependence. Some authors have divided its electrochemical behaviour into five distinct pH regimes with different reaction mechanisms, few of which are relevant to the implant designer [21].
W forms a stable oxide layer of WO3 in acidic solutions (pH < 4) [22–31]—this is well outside the operating pH range of typical medical implants (except gastric implants).
In solutions of neutral and alkaline pH values, the WxOy surface species form soluble tungstate ions (often represented as 'orthotungstate'  [32]), which results in the continual dissolution of surface material; these are the products of W oxide dissolution as well as active W metal dissolution at higher pHs [23, 33–35]. All authors agree that in neutral to alkaline aqueous environments, the oxide phase on the metal surface hydrates, and then passes into solution as these hexavalent tungstate ions [18, 33, 34, 36–42]. Weidman's chronoamperometry measurements at 1.0 V sweeping from pH 0.5 to 13.0 presented sharp increases in current densities between pH 4–6, indicating that there is no passivation occurring via oxidation at higher pH values [22]. Heumann and Stolica [36] used weight loss measurements and coulometric methods to determine that the valence of dissolved W was indeed 6.0, agreeing with earlier work regarding W solubility.
[32]), which results in the continual dissolution of surface material; these are the products of W oxide dissolution as well as active W metal dissolution at higher pHs [23, 33–35]. All authors agree that in neutral to alkaline aqueous environments, the oxide phase on the metal surface hydrates, and then passes into solution as these hexavalent tungstate ions [18, 33, 34, 36–42]. Weidman's chronoamperometry measurements at 1.0 V sweeping from pH 0.5 to 13.0 presented sharp increases in current densities between pH 4–6, indicating that there is no passivation occurring via oxidation at higher pH values [22]. Heumann and Stolica [36] used weight loss measurements and coulometric methods to determine that the valence of dissolved W was indeed 6.0, agreeing with earlier work regarding W solubility.
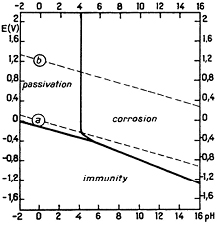
According to Pourbaix, the borderline conditions of corrosion/passivation occur between pH 4–6 (figure 1). Above pH 6, general corrosion will occur, with hydrogen evolution in the absence of oxidising species, and without hydrogen evolution in their presence. The tendency for corrosion rises with increasing potential [19], as may occur when W is used as stimulating electrodes. Weidman reports that at current densities exceeding 0.1 mA cm−2 there is a breakdown in the passivated surface regardless of pH, even in acidic zones [22]—a possible alteration to Pourbaix's initial diagram. This acid-soluble  ion has been proposed by others [43, 44].
ion has been proposed by others [43, 44].
Figure 1. Pourbaix's theoretical EH/pH diagram for W in H2O at 25 °C [20].
Download figure:
Standard image High-resolution imageThe original Pourbaix diagram applies to pure water at 25 °C, not the physiological environment, and thus should be used with caution. His analysis also ignores the numerous complexes W forms—these include hydrochloric complexes of trivalent W, hydrofluoric and oxalic complexes of tetravalent W, and cyanide complexes of pentavalent W. Some tungstates of alkali metals are soluble, while others are not [45].
A useful improvement to the diagram was generated computationally by Patrick for W in phosphate-buffered saline (PBS) using the CorrosionAnalyzer 1.3 software—approximately 60 solid, aqueous, and gaseous species were considered [13]. It is redrawn here for clarity with dominant species labelled (figure 2). Similar conclusions can be made: W is clearly a base metal, with a stable domain in the physiological pH range (x–y) (5.6 to 9.0 [14]) only outside of the water window (a–b).
Figure 2. EH/pH Diagram for W in PBS based on Patrick's work [13].
Download figure:
Standard image High-resolution imageHeumann et al [18] still refer to tungsten's 'protective film' of WO2—what may be termed a protective or passive oxide film may continually form at all pH values, inhibiting dissolution to some degree [46]. However, the long-term behaviour remains as continual dissolution into soluble hexavalent W6+. With this continual dissolution process in neutral solutions, W is unsuitable for use as a chronic implant material.
2.2. Rates of corrosion
Lassner and Schubert [3] report corrosion of metallic W at the rate of 3.8 µg m−2 h−1 at 38 °C in distilled water. Gmelin [5, 7] reports corrosion rates of 0.83 µg m−2 h−1 in 3% NaCl solutions and 89.2 µg m−2 h−1 in 10% KOH solutions, both aerated and agitated at 20 °C. Of more significance, W exhibits a corrosive weakness to hydrogen peroxide [46]. Peroxide solutions dissolve W without inhibition, and the rate of dissolution increases linearly with H2O2 concentration [7, 47]. Hydrogen peroxide etches W along crystal planes (1 0 0), (1 1 1), and (1 1 2), and further at subgrains and wide-angle boundaries. It rapidly dissolves powders [3], as well as bulk billets of compact W, with dissolution rates of 1.6 mg l−1 min−1 reported in aqueous solutions of peroxide at a concentration of 14 g H2O2/l over 180 min at 20 ± 1 °C [48]. This peroxide effect has also been noted by Patrick et al [13] in a study on W microelectrodes, where an increase in corrosion rates on the range of two orders of magnitude in benchtop studies with 30 mM H2O2 in PBS versus no peroxide was reported.
While the relation between these experimental concentrations and physiological concentrations is debatable, it is worth mentioning given that peroxide is readily produced in the body's foreign-body immune response near implants. In addition to peroxides, reactive oxygen species such as the superoxide anion and the hydroxyl radical are rapidly released by immune cells including microglia in the central nervous system (CNS) [13, 49] and neutrophils in the blood [50] in events termed 'oxidative bursts' [51–53]. In non-acute animal studies, Prasad reports the presence of activated microglia near W electrode tracks in all cases, indicating a neuroinflammatory response regardless of post-implantation survival times and electrode performance [54]. These species would no doubt accelerate the corrosion of W through the oxidation to its soluble hexavalent form.
2.2.1. Chemical reactions.
The overall six-electron stoichiometric equation for neutral to basic solutions is as follows [20, 34]:
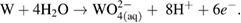
This overall anodic equation occurs step-wise as below, elucidating the rate-determining step, which is first-order with respect to OH− [3]:
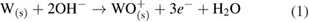
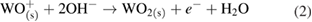
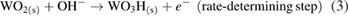
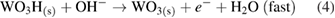
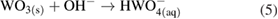
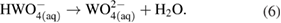
The reduction of water/oxygen and of peroxide are the likely in vivo cathodic reactions [13]:
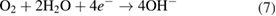
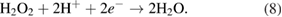
Alternative anodic pathways are reported [22]. Older publications state W2O5OH(s) + W2O5(s) → W2O5–OH–W2O5(s) as the rate-determining step [33], but this was superseded by newer data consistent with reaction (3) followed by the rapid OH− ion-assisted dissolution of WO3H(s) to the  species [46]. Works quoted by Gmelin state that the W2O5 referred to in older literature does not exist and in fact corresponds to W20O58 (or WO2.9) [19]; this is supported by more recent literature. The above series of equations thus corresponds to the oxidative series of W: W → W3+ → W4+ → W5+ → W6+.
species [46]. Works quoted by Gmelin state that the W2O5 referred to in older literature does not exist and in fact corresponds to W20O58 (or WO2.9) [19]; this is supported by more recent literature. The above series of equations thus corresponds to the oxidative series of W: W → W3+ → W4+ → W5+ → W6+.
Anodic polarisation of W in acidic media (or reduction from WO3) will present distinct stages through the formation of all stable stoichiometric oxides, each with a distinct colour regime:
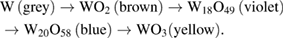
Given tungsten's particular weakness to peroxide dissolution, this chemical reaction pathway will be mentioned:
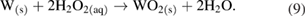
Two pathways have then been proposed, resulting in various compounds referred to as peroxotungstates, pertungstates, or pertungstic acid [3]:
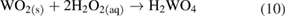
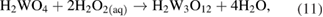
and
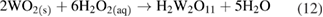
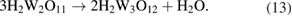
Pourbaix represents these reaction products as  ions; this is an uncommon usage [20].
ions; this is an uncommon usage [20].
2.3. Electrochemistry
The open-circuit (or Ecorr corrosion potential) of W was found to be approximately −100 mV at pH 7 with respect to the standard hydrogen electrode [42], and decreases by 43 mV per pH increase [18]; as the pH increases, the corrosion potential shifts to less noble potentials [23, 46]. In these studies, the anions of the buffer solutions ( ,
,  ,
,  , etc) were found to influence the electrochemistry. Kelsey [34] found the anodic Tafel slope at all concentrations of hydroxide to be 0.14 V decade−1.
, etc) were found to influence the electrochemistry. Kelsey [34] found the anodic Tafel slope at all concentrations of hydroxide to be 0.14 V decade−1.
Readers with deeper interest in the electrochemical behaviour of W electrodes at anodic potentials, at wider pH ranges, and with results from cyclic voltammetry, linear sweep voltammetry, potentiodynamic polarization experiments, and analyses of the AC impedance spectra of W oxide films should consult the excellent papers by El-Wakkad [42], El-Basiouny [39, 42, 43, 55], Heumann and Stolica [18, 36], Wiedman [22], and Anik et al [21, 23, 30, 56, 57], amongst others.
3. Medical implant corrosion
Instances of corrosion of W medical implants in the literature are summarised here. Examples are primarily of embolisation coils and neural microelectrode probes. Implant functionality depends on the integrity of the bulk metal form; the abiotic failure of medical implants via metallic corrosion inhibits their chronic use.
3.1. Corrosion of tungsten coils
Tungsten coils have been used to perform transcatheter embolisations of pathological blood vessels due to their high radiopacity (W behaving as a very effective x-ray absorber due to its high density [3]) as well as its thrombogenicity [58, 59]. The W 'Mechanically Detachable System' (MDS) coils (Balt Extrusion, Montmorency, France) are an example of a once commonly used system [16, 60–62].
Rabbit studies of W coils implanted for 3–6 months for embolisation exhibited corrosion, with black particles of metal spectroscopically determined to contain W and Fe found in the adventitia of the aneurysm and outside the lumen, distant from the W coils in phagocytic cells, and also in fibroblasts and spindle cells [63]. The iron is hypothesised by Peuster [62] to be not from coil manufacturing contamination but agglutinated blood on explantation. A similar rabbit study by Peuster et al [64] demonstrated a 3.5-fold increase in serum W concentration as early as 15 min post-implantation.
In 1998, a study by Weill et al [16] first presented the 'disturbing' finding of in vivo W corrosion in five patients with decreasing levels of radiopacity in skull plain x-ray films of MDS embolisation spirals 33–45 months post-implantation for intracranial aneurysm or dural fistula by venous approach. W blood serum levels in patients were 10–50 times the population baseline, proportional to the length of coil implanted. Additionally, elevated blood serum levels in one patient three months post-implantation (4.95 µg l−1 versus baseline <0.1 µg l−1 from pooled population samples [16, 65]) without a demonstrably noticeable decrease in radiopacity indicates that corrosion occurs earlier than is radiographically detectable.
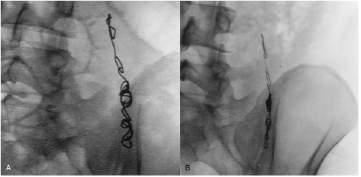
Barrett et al [66] demonstrated similar results for W MDS coils used for endovascular embolisation of varicoceles in the scrotum, presenting radiographic evidence of W resorption in 4 out of 18 patients (figure 3), and elevated W blood levels in 18 out of 19 patients (mean 40 months post-implantation). Bachthaler et al [60] reported W coil corrosion for intracerebral aneurysms and dural fistulae 26 months post-implantation in 9 out of 14 patients also by plain radiography, with two patients displaying complete coil resorption. W blood levels were elevated in 6 out of 14 patients, and urine levels were elevated in all. Others have reported similar radiographic and blood serum results with the use of W embolisation coils in intracranial aneurysms, abdominal aortic stent grafts, spermatic vein varioceles, oesophageal and gastric varices, and other pathological blood vessels (figures 4 and 5) [61, 67–70].
Figure 3. Endovascular embolisation of varicoceles in the spermatic vein with W coils. (A) Image obtained during procedure; (B) 57 months later, with clear reduction in the substance, thickness, and overall volume of the coil [66].
Download figure:
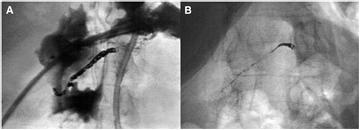
Standard image High-resolution imageFigure 4. W coil embolisation of the right hepatic artery for massive haemorrhage after stomach resection. (A) Image obtained immediately post-procedure; (B) 35 months later, with clear decrease in metal density [60].
Download figure:
Standard image High-resolution imageFigure 5. Chest radiographs in a 3-month-old girl after surgical correction of multiple ventricular septal defects and coarctation repair. (A) One d after coil embolisation of one aorto-pulmonary collateral with three 3.50 mm W coils; (B) after 11 months; and (C) after 28 months. All coils are completely corroded after 28 months [68].
Download figure:
Standard image High-resolution imageIn a metallographic analysis, Peuster's SEM examination of the W coils explanted from the rabbit subclavian arteries after four months demonstrated a clear change from a smooth surface of the control with a uniformly circular cross-section to surface roughening, pitting, and crevice corrosion in the implanted coils [64].
Various causes for this corrosion have been proposed in the literature. Weill et al [16, 63] blame the metallic impurity of the MDS coils. This was disproven by studies that spectroscopically verified the purity of the W pre-implantation (>99.9% by mass) [60–62, 71]. Barrett et al [66] attribute the degradation due to mechanical effects of percussive systolic–diastolic blood flow on the spirals, but this explanation was nullified when corrosion was observed by radiography in venous fistula drainage pathways with no percussive flow [16], as well as in cannulae of the spermatic vein [66]. Other cases have been reported [72].
While it may be possible that mechanical factors such as percussive flows may accelerate corrosion, as discussed at length previously, the underlying reason for W coil corrosion and eventual failure is known, i.e. the unfavourable thermodynamics of metal corrosion within the implant environment of the human body, i.e. the propensity for metallic W to dissolve into tungstate at pH 7.4 and 37 °C [19, 20]. A mass dissolution rate of 29 µg d−1 was reported for W coils [73], but the proper rate relation to exposed surface area is not explored. Peuster [74] reports localised acidosis in occluded vessels due to metabolic processes and lack of oxygen carriers leading to stable passivating oxide layers on the W coil. However, reports of coil dissolution refute this—evidence of a local pH less than 4 (Pourbaix's upper limit for stable oxide formation) was not reported.
The general consensus of authors is that the use of W embolisation coils should cease [16, 60, 61, 63, 66–69, 74]. Recanalization as the failure mode of these W embolisation coils occurred in 58.3% of cases in [68], and was reported by others as proportional to the degree of corrosion [16]—an unacceptable risk for patients.
3.2. Corrosion of tungsten microwires
Long-term corrosion resistance is necessary for extracting stable signals from chronic implanted neural electrodes. Additionally, for single neuron recordings, electrodes must be less than 200 µm from the soma to record the action potential [75]—at such microscopic scales, even the slightest corrosion will cause device failure.
W microwires have been used in animals for this purpose for almost 60 years [54, 76–79]. High-purity wire is readily available, and the metal is stiff and robust enough to be readily fabricated into fine tips capable of performing single neuron electrophysiological recordings [76]. Commercial W microwires of 99.95% purity are available at various diameters (~50–125 µm). These can be sharpened to tips of ultramicroscopic dimensions (0.5–0.05 µm) by electrolytic etching, with oscillatory dipping into saturated KNO2 or another etching solution while passing a current [80–83]. Tungsten's high melting point and low coefficient of thermal expansion enable it to be easily encapsulated within glass capillaries [84]; other encapsulants such as polyimide [82, 85, 86] and Parylene C have also been used. Exposed tips may be electroplated with gold, platinum, or other metals to decrease the electrode impedance [84, 87].
With the same technology, W has also been used to form recording electrode arrays [85]. These are termed linear microelectrode arrays, microelectrode arrays, microwire arrays, or floating microelectrode arrays. There are many examples of recording sites in commercial and non-commercial research utilising intracortical microelectrode arrays of W microwires [17, 85–91].
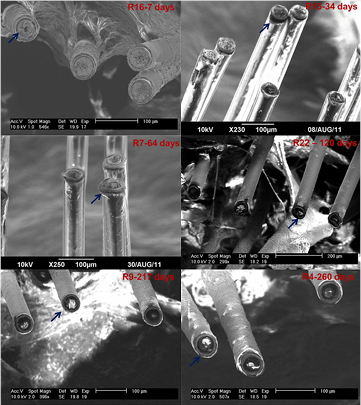
Unfortunately, in chronic applications, corrosion is again a limiting factor for their success as implants. Prasad et al [54] note that while W microwires are able to initially provide stable recordings, they fail to do so for years, reporting roughening of the surfaces of their 50 µm diameter microelectrodes two h after implantation, recession and cavitation of surfaces after two weeks to 16 d, and eventual cracking, pitting, hole-formation, and deep recession of the electrodes into the insulation in months following (figure 6). Williams [85] reports W microwire electrodes of 35 µm diameter (unsharpened) having viability of continuous recordings from guinea pig tests of only five weeks or less. Sanchez et al [87] reportes corrosion of W microwires after four weeks of in vivo implantation. Others report similar degradation of recording surfaces of W microwires [87].
Figure 6. Post-explantation SEM images of 16-channel tungsten microwire arrays with 50 µm diameter electrodes coated with 10 µm thick polyimide insulation. The images depict the progression of electrode recording site corrosion and deterioration for increasing implant durations in different rats labelled by 'R' (R16: 7 d; R15: 34 d; R7: 64 d; R22: 122 d; R9: 217 d; R4: 260 d). The arrows (original authors' addition) indicate examples of polyimide delamination and electrode recording site corrosion for individual electrodes [54].
Download figure:
Standard image High-resolution imageA very thorough analysis of this corrosion phenomenon was performed by Patrick et al [13] on commercial 50 µm diameter W microwires (California Fine Wire) encased in epoxy (Epotek 302-3M). Reported bench-top corrosion rates were 300–700 µm yr−1 for pure W in 0.9% PBS, and 10 000–20 000 µm yr−1 for Au-plated W (gold deposited circumferentially) in 0.9% PBS + 30 mM H2O2 (the latter to simulate the highly oxidative environment of post-implantation inflammation). Assuming 100% W, ideal cylindrical geometry, full density, and ρw = 19.25 g cm−3, this corresponds to 0.45–1.1 µg yr−1 and 15–30 µg yr−1, respectively. No data on pure W in PBS/H2O2 was offered.
Simultaneous in vivo studies in adult male Sprague–Dawley rats implanted cranially with Au-plated W reported corrosion rates of 100 µm yr−1 (0.151 µg yr−1) with the difference hypothesised to be due to the formation of an oxygen-blocking biofilm [13].
From purely visual analysis, in the PBS-only samples, the electrode surfaces polished to 1 µm smoothness displayed roughening after 1 d, pitting after 2 d, and full recession from the initial surface after 6 d to a depth of >4 µm, similar to the reports by Prasad. In the in vivo studies, the W receded within its insulation 24 ± 8 µm after 87 d of implantation (figure 7). Similar results were reported by others (figure 8). No oxide layer was present in either case, contradicting claims of oxide passivation due to localised acidosis.
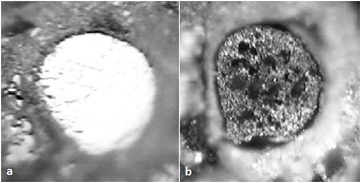
Figure 7. Optical photographs of 50 µm nominal diameter W electrode. (A) Before immersion in PBS (polished); (B) after 23 d of immersion, with evidence of corrosion: surface roughening, pitting, and dark oxidic patches [13].
Download figure:
Standard image High-resolution imageFigure 8. SEM images of recording tips of 50 µm diameter W microelectrodes encased in polyimide implanted for 7 d. L: pre-implant; R: post-implant. Corrosion has occurred, indicated by a darkening, loss of volume, and a change in surface morphology [92].
Download figure:
Standard image High-resolution image3.3. W alloys in medical implants
In cardiovascular medicine, permanently implanted bare-metal stents are used to restore luminal patency in patients with obstructive coronary lesions. A common stent platform alloy used is L605 cobalt–chromium alloy with 15% W wt% [93]. No corrosion or failure has been reported.
In prosthodontics, W is used as a component of cobalt–chromium alloys, e.g. Wirobond® C (BEGO GmbH, Bremen, Germany) is 5% w/w, containing also Co (61%), Cr (26%), Mo (6%), and small amounts of Fe, Ce, and C [94]. No corrosion or failure has been reported.
In orthopaedics, cobalt-based wrought alloy ASTM F90 is used for total joint replacement; its composition is Co–20Cr–15W–10Ni. W composition is 14–16% (wt%) [95]. It is a popular orthopaedic alloy [96]. No corrosion or failure has been reported.
4. Thin film tungsten
As designers think about smaller implanted devices, they will consider using thin-film methods. At present we do not know of any medical implants with thin tungsten films. However, aqueous corrosion studies of tungsten thin films have been performed, and this will be briefly reviewed here.
It is not a safe assumption that metals will exhibit identical behaviour, including corrosion, in thin film as they do in bulk; differences in grain size and orientation, pinhole formation, pitting, porosity, and other phenomena exist between the two states [97, 98].
Most discussions in the literature regarding the behaviour of W thin films in aqueous solutions are from the integrated circuit (IC) industry, and their attempts to remove oxide layers from tungsten ohmic contacts are through vias using chemical mechanical polishing [28, 29, 46, 99–102]. There is some relevance for the implant designer.
Kneer used low pressure chemical vapour deposition (LPCVD) to deposit 0.8 µm thin films of tungsten via WF6 reduction onto silicon substrates using a 50 nm TiN adhesion layer. X-ray diffraction patterns were identical to that of W powder standards. In the absence of peroxide the values for icorr in the pH range 2–9 were approximately 1 µA cm−2, corresponding to a dissolution rate of 0.01 nm min−1 for tungsten dissolving to the hexavalent species. Unsurprisingly, peroxide solutions rapidly etched through the film, with complete dissolution in 10 min at 4–5% concentrations. Field emission scanning electron microscope (FESEM) micrographs depicted clear surface etching [46].
DC potentiodynamic polarization results indicate that in the absence of H2O2 an oxide layer forms, which decreases tungsten dissolution in the pH range from 2 to 11. This oxide layer provides the best passivation at pH 2, less protection at pH from 4 to 9, and none as the solution conditions become more alkaline [46]. This concurs with earlier bulk tungsten analysis.
Yin et al provide data on the dissolution of thin film W oxides in deionized water, reporting dissolution rates of about 0.2–0.5 nm d−1. Furthermore, in the pH range of 7.4–8, they report an order of magnitude higher dissolution rates (µm h−1) for W that has undergone physical deposition methods (sputtered) compared to chemical (chemical vapour depostion (CVD) W) in both deionised (DI) (water) (4 ± 1 × 10−3 versus 7 ± 2 × 10−4) and in Hank's solution (8 ± 2 × 10−3 versus 7 ± 2 × 10−4) [97]. Given a typical W metallisation layer of 200 nm thickness, theoretically, after ~400 d it would completely dissolve.
Some other issues for the implant designer to consider at thin film scales are electromigration, solid state diffusion, substrate adhesion, and the effects of chronic implantation on solid state material properties. Tungsten reacts with silicon at 600 °C (a temperature it reaches during physical vapour deposition methods), forming silicide compounds that will affect electrical properties. This is another point to consider.
5. Toxicity of tungsten
In light of the previous corrosion analysis and with the knowledge that tungsten implants will invariably shed tungstates into the body, it is worth examining the data on its toxicity to inform future implant designers on its further use.
Concern for W toxicity began with epidemiological studies in Dayu County of Jianxi Province, China, where several W mines are located, which revealed breast cancer mortalities to be 13.8 times higher in men and 2.5 times higher in women compared to the national average (1985) [103]. Furthermore, childhood acute lymphocytic leukaemia (ALL) clusters were reported in Fallon, Nevada, US where Centre for Disease Control (CDC) studies on toxicology and carcinogenesis showed elevated tungsten levels in drinking water and in the body burden in residents [104–107]. Further childhood ALL clusters were then reported in Sierra Vista, AZ and Elk Grove, CA, both of which were also rural desert towns in close proximity with tungsten mines, processing operations, and military bases, with dendrochemistry consistently reporting significant increases in W compared to older wood [108, 109].
Acute tungsten intoxication was reported in a French soldier who had consumed 250 ml of wine though the barrel of a gun (inadvertently consuming 385 mg of W used as a steel hardener). High concentrations of tungsten were measured in his serum, urine, and stomach contents. Symptoms included seizure, loss of consciousness, and tubular necrosis [110]. These reports were questioned by others who attributed the soldier's symptoms to 1,3,5-trinitro-1,3,5-triazine (also known as hexogen, cyclonite, or RDX) a military explosive compound and known epileptogenic, given the routine use of 25–80 g powdered tungsten metal by mouth as a substitute for barium in radiological examinations. This was rejected in turn [111]. Cell culture studies have been performed since, with the LD50 reported as being 50 mg ml−1 for human pulmonary arterial endothelial cells, 100 mg ml−1 for human pulmonary arterial smooth muscle cells, and 1000 mg ml−1 for human dermal fibroblasts [73]. Another study reported markedly elevated tungsten concentrations in a culture medium of 120 000 times higher than baseline serum levels (0.4 µg l−1) with in vitro cultivation using human vascular endothelial cells, human fibroblasts, and human smooth muscle cells; no toxicity was reported [62]. Previous tungsten coil degradation studies in humans reported absence of toxicity resulting from corrosion in adult and paediatric patients despite elevated serum tungsten levels [16, 61, 67, 73, 74].
The role of W in heavy metal disease has also been explored; while W is thought of as a hard metal, it has been reported that the disease does not relate to the levels of W in blood, urine, pubic hair, and toe nails [112]. Concentrations of tungsten in the blood, toenails, urine, and pubic hair, is reported to be elevated even in asymptomatic workers exposed to tungsten dust as compared to normal subjects [113]. In similar data on other occupational exposures in plants with W powder metallurgy, workers chronically exposed to W air concentrations of 5 mg m−3 (0.7 ppm) developed no pneumoconiosis [104].
A synergistic toxicity with cobalt was first reported by Lison [114], with the combination more toxic towards murine macrophages than Co alone. Similarly, studies of military munitions of heavy metal tungsten alloys consisting of W, Co, Ni, and Fe showed the compounds to be genotoxic, inducing neoplastic transformation of human osteoblast cells to the tumorigenic phenotype [115]. Other studies on military rWNiCo (reconstituted) also show tumorigenic effects [116]. Kalinich [117] performed similar studies whereby weapons-grade tungsten alloy (91.1W–6.0Ni–2.9Co) embedded intramuscularly in F344 rats rapidly induced metastatic high-grade rhabdomyosarcomas. Kalinich, as did Miller, argues that there exists a synergistic toxicity of W with other hard metals, and suggests this may have occurred environmentally at Fallon [106]. Others too have reported cytotoxicity of the W–Co combination [118].
As described earlier, these metal combinations (though in different proportions) are present in stents, and prosthodontic and orthopaedic implants, such as ASTM F90 (Co–20Cr–15W–10Ni) [93–96]. No such carcinogenicity has been reported for these applications.
Various animal studies are available: the i.p. LD50 value of tungsten metal powder in rats is reported to be 5 g kg−1 (0.03 mol kg−1) [104]. Others have reported for sodium tungstate the oral and i.v. LD50 values of 1928.4 and 61.0 mg kg−1, respectively, whereas in mice, the LD50 values were found to be 1904.1 and 107.1 mg kg−1, respectively [119]. These LD50 values indicate that tungsten has a rather low toxicity. Orally administered tungstate has been reported to correct hyperglycaemia in insulin- and non-insulin-dependent models of diabetes in rats and mice. Undesirable side effects were not observed, and there was no evidence of intolerance after prolonged use [120–125]. In rabbits a 30-fold increase in W serum concentration four months after implantation did not lead to any signs of clinical or histopathological toxicity [64].
There is small discussion on the effect of speciation on toxicity with variable animal studies, suggesting that sodium metatungstate is significantly more toxic than sodium tungstate [126–128]; given the dominant presence of tungstate as the soluble species, it is of small relevance.
The literature is varied, and clear conclusions are difficult to draw. The interested should refer to deeper explorations [129–132].
6. Conclusion
The use of pure tungsten in chronic medical implants should be avoided. It has several properties that have made its use attractive, and thus has a long history for implants in several medical fields. However, poor corrosion resistance of the pure metal in aqueous solutions makes it unsuitable for use in chronic devices. Unlike many other metals, tungsten neither maintains a noble metallic surface, nor does it form a protective passivation layer of tungsten oxide. At the body's pH of 7.4, the solid WO3 layer will hydrate, and dissolve away into the soluble hexavalent anion, W6+ or  . This conclusion does not refer to its use within implantable alloys.
. This conclusion does not refer to its use within implantable alloys.
Corrosion has been reported in vitro in distilled water, in PBS, in peroxide-rich PBS, and in vivo with embolisation coils and micro-needle neural probes. The associated emission of tungstate ions has been observed, and elevated blood serum levels have been measured in various animal studies, and in various human patients. Its toxicity is also currently under investigation, with some reports of acute poisoning and long-term genotoxicity; however, as these investigations continue it remains the case that its poor corrosion behaviour leading to implant failure is the primary reason for avoiding its use.
Given the wide range of materials available to medical implant designers, the authors recommend alternative materials.
Acknowledgment
This work has been funded by the Wellcome Trust (contract ref: 102037/Z/13/Z) and The Engineering and Physical Sciences Research Council (EPSRC) (grant ref: NS/A000026/1) as part of the CANDO Innovative Engineering for Health project.
Declaration of interest
The authors confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Appendix
A.1. Medical device recalls
An exploratory search for 'TUNGSTEN' in the US Food and Drug Administration (FDA) 'Medical Device Recalls' database was hoped to reveal other implant failures. Three cases were found:
- 1.A Class I recall in 2011 (Z-1865-2011) [133] of Axxent FlexiShield Mini, a radiotherapy beam-blocker device made of a circular silicone rubber pad containing tungsten particulate. The pad is 12.7 cm in diameter and 0.1 cm (1 mm) thick. Recall reason: '[The] product may shed particles identified as tungsten'.
- 2.A Class II recall in 2015 (Z-0120-2016) [134] of Medtronic Pipeline Flex Embolisation Device. Made of 75% cobalt–chromium/25% platinum–tungsten. Recall reason: 'The firm is recalling Pipeline and Pipeline Flex Embolisation Devices from US since the devices were shipped with an EU version of the Instructions for Use'.
- 3.A Class II recall in 2010 (Z-1760-2010) [135] of Boston Scientific Back-Up Meier Steerable Guidewires, made of a stainless-steel, PTFE-coated proximal shaft, and a distal tip of radio-opaque gold-plated tungsten spring coil wound around a stainless-steel inner core. Recall reason: 'Through their internal inspection process, they identified that the polytetrafluoroethylene (PTFE) coating on the gold plated distal coil of the Back-up Meier Steerable Guidewires of the identified lots/batches have the potential for PTFE delamination'.
Similarly, a search for 'TUNGSTEN' in the Australian Therapeutic Goods Authority (TGA) 'System for Australian Recall Actions' database reveals one case:
- 1.A Class II recall in 2015 (RC-2015-RN-00263-1) [136] or AngioDynamics Morpheus Smart PICC CT (central venous catheters); tungsten content unclear. Recall reason: 'Based on a review of complaints, AngioDynamics has determined that the power injectable PICC catheters contained in these kits exhibit a higher than anticipated rate of extension tube fracture or leakage'.
Similar searches in the UK The Medicines and Healthcare Products Regulatory Agency (MHRA) 'Alerts and Recalls for Drugs and Medical Devices' database [137] and EU European Medicines Agency (EMA) 'Product defects and recalls' database revealed no similar results.
None were corrosive in nature.