Abstract
Anthropomorphic phantoms used for radiation dose measurements are designed to mimic human tissue in shape, size, and tissue composition. Reference phantoms are widely available and are sufficiently similar to many, but not all, human subjects. 3D printing has the potential to overcome some of these shortcomings by enabling rapid fabrication of personalized phantoms for individual human subjects based on radiographic imaging data. Objective. The objective of this study was to test the efficacy of personalized 3D printed phantoms for charged particle therapy. To accomplish this, we measured dose distributions from 6 to 20 MeV electron beams, incident on printed and molded slices of phantoms. Approach. Specifically, we determined the radiological properties of 3D printed phantoms, including beam penetration range. Additionally, we designed and printed a personalized head phantom to compare results obtained with a commercial, reference head phantom for quality assurance of therapeutic electron beam dose calculations. Main Results. For regions of soft tissue, gamma index analyses revealed a 3D printed slice was able to adequately model the same electron beam penetration ranges as the molded reference slice. The printed, personalized phantom provided superior dosimetric accuracy compared to the molded reference phantom for electron beam dose calculations at all electron beam energies. However, current limitations in the ability to print high-density structures, such as bone, limited pass rates of 60% or better at 16 and 20 MeV electron beam energies. Significance. This study showed that creating personalized phantoms using 3D printing techniques is a feasible way to substantially improve the accuracy of dose measurements of therapeutic electron beams, but further improvements in printing techniques are necessary in order to increase the printable density in phantoms.
Export citation and abstract BibTeX RIS
1. Introduction
Anthropomorphic phantoms typically use reference human anatomy and are constructed using tissue-substitute materials to mimic both the internal and external geometry of the human body. The major benefit of anthropomorphic phantoms over other phantoms, such as water-box tanks, is the increased accuracy in the modeling of external human shape and internal tissue heterogeneities (DeWerd and Kissick 2014). Simplistic anthropomorphic phantoms comprise humanoid exterior geometry, homogeneous internal material, or simplified internal heterogeneities. In recent decades, anthropomorphic phantoms have become much more realistic in geometric detail and material composition (Winslow et al 2009). Most of the current state-of-the-art in anthropomorphic phantoms utilize average or reference anatomic specifications, such as those described in Zankl et al (2018).
3D printing uses additive fabrication methods to construct three-dimensional structures (Wong and Hernandez 2012). This emerging technology has seen increased utilization in radiation oncology, with applications including the development of compensator blocks and immobilization devices (Avelino et al 2012, Ju et al 2014, Tino et al 2019a). In recent years, there has been increasing interest in using 3D printing to create personalized phantoms, e.g. to create positive molds for custom geometry dosimeters (Bache et al 2015), to fabricate an anthropomorphic reference phantom for quality assurance in photon radiotherapy (Ehler et al 2014) and imaging studies (Gear et al 2014, Filipou and Tsoumpas 2018). 3D-printed phantoms ideally combine the flexibility, high spatial resolution, and realistic tissue-substitute materials (Price et al 2020).
While considerable progress has been made in improving anthropomorphic reference phantoms' capability to mimic internal and external anatomy (Price et al 2020), reference phantoms are not always adequate to mimic anatomical features of individual patients (Tino et al 2021). Some peculiar features are either averaged out or omitted in the fabrication process due to the use of reference anatomy as a design basis and the difficulty of fabricating such features using traditional manufacturing methods. Examples of patient-specific anatomical features that are not accurately modeled by traditional anthropomorphic phantoms include surgical amputations, implanted devices, growth abnormalities, sinus cavities, and bony processes such as the petrous ridge of the temporal bone of the cranium (Mittauer et al 2020). Kadoya et al (2019) reported liquid plaster leakages from the thinly printed walls of sinus cavities. In cases where a patient's anatomy is highly unique, it may be necessary to create a personalized phantom to confirm and measure radiation dose distributions. This is especially important for charged particle therapies, where external contours and internal heterogeneities can have a significant impact on dose distributions. Personalized phantoms designed using patient imaging data could address such needs (Zhang et al 2019).
The goal of this work is to confirm the feasibility of personalized, 3D printed phantoms over reference phantoms for dose measurements in charged particle radiation fields. We tested and compared the dosimetric performance of novel personalized and well-characterized reference phantoms using treatment planning calculations and measurements for 6–20 MeV electron-beam irradiations of the cranium.
2. Methods
2.1. Comparison of printed and molded anthropomorphic phantom slices
To increase confidence in our methods, we first sought to reproduce dosimetric results obtained with established methods that include a molded anthropomorphic phantom. To accomplish this, we acquired CT images of a well-characterized and widely-used molded anthropomorphic phantom, then we designed and replicated of a portion of the molded phantom using 3D printing methods. To compare the dosimetric properties of the molded and 3D-printed phantoms, we irradiated them with identical electron beams.
To create the 3D-printed reference phantom, we selected a pediatric anthropomorphic phantom (Model 706-C ATOM® Pediatric 10 year old Dosimetry Phantom, S/N 706-L1652, CIRS Inc., Norfolk, VA) for our design basis. More specifically, we selected a sagittal section of the cranium of this phantom as our reference slice. A pediatric phantom geometry was selected for this comparison because its smaller size yielded fewer post-printing warpage issues that a larger adult-size phantom.
The phantom head was imaged using a clinical CT scanner (GE Lightspeed RT16, S/N 1255068, GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) following clinical practices for radiotherapy treatment planning (e.g. all CT scans in this work were acquired with 120 kVp tube potential, 400 mA tube current, 2.5 mm slice thickness, and 40 cm scan length). The image set was exported to the radiation therapy treatment planning system (TPS) (Pinnacle 3 Treatment Planning System Version 9.8, Philips Healthcare, Andover, MA), where the exterior geometry of the reference slice was contoured to delineate the exterior surface of the slice.
The various anatomical structure contours (e.g. skin, bone, cranial cavity) were then extracted from the TPS and converted, using in-house software, into standard tessellation language (STL), a file format widely utilized in 3D printing. The reference slice was then printed in-house using a fused deposition 3D printer (AW3D XL, AirWolf 3D, Costa Mesa, CA). The slice was printed using polylactic acid (PLA) (Prototype Supply 3 mm PLA filament, Lot 20140601AD, ToyBuilder Labs, Pasadena, CA) as the printer filament. PLA was selected to represent all tissue, including bone, for this work based on the findings of Halloran (2015). Because its mass density and mass stopping power are similar to those of soft tissue, PLA is an excellent substitute for soft tissue, but less so for bone, mainly because of bone's relatively higher mass density. Due to printer material limitations, PLA was used as a substitute for both bone and soft tissue. We selected printer infill settings following Halloran (2015) and Madamesila et al (2016).
The irradiations were delivered with an electron linear accelerator (Varian Model 21EX 6/18, S/N 1251, Palo Alto, CA) and absorbed dose distributions were measured using radiochromic film (EBT3 Gafchromic Film, Ashland Inc., Covington KY, Lot #06051403). Films were calibrated at a distance of 100 cm using a 6 cm electron cone and 4 cm lead insert for each available electron energy. Calibration irradiations ranged from 0 to 300 Monitor Units (MU), delivered in 50 MU increments. Both the molded and printed phantom slices were irradiated using broad electron beams perpendicular to the slices (figure 1). The slices were irradiated at 6, 9, 12, 16, and 20 MeV nominal electron beam energies. Film was placed between each slice and distal backscatter material to measure the penetration dose.
Figure 1. A graphic of the setup used in performing the irradiations of the printed and molded reference slices. The phantom slice (blue) was placed atop the sheet of radiochromic film (green) and irradiated with a broad, electron beam (arrows). A water equivalent plastic slab (light gray) was placed distal to the film as a backscatter medium.
Download figure:
Standard image High-resolution imageThe irradiated films were allowed to age for 48 h after exposure to permit any further changes in color density prior to digitization (Niroomand-Rad et al 1998). They were digitized with a flatbed scanner (Expression 10000 XL, Epson America, Inc., Long Beach, CA). The digitized film responses were converted to dose distributions and analyzed using commercially available quality assurance software (RIT Radiation Therapy QA and Diagnostic Imaging QC Software, Radiation Imaging Technology, Colorado Springs, CO). We used the gamma index analysis to compare dose distributions and we applied the widely used criteria of 3% dose difference (DD) and 3 mm distance to agreement (DTA). The dose distributions obtained using the molded phantom slice served as the reference or 'gold standard' data.
At each electron beam energy, the dose distributions obtained using molded and printed slices were registered and compared to determine differences in the 2D absorbed dose distribution where the beam exited the slice. Three different regions of interest (ROIs) were examined to determine the dosimetric differences: the entire irradiated slice, an area of bone-soft-tissue interface, and a homogeneous brain tissue region (figure 2).
Figure 2. The regions of interest (ROI) used for the gamma index analyses of the molded and printed slices. The red bounding boxes indicate the three ROIs used in the comparison of the molded and printed slices. ROIs 1, 2, and 3 examine the entire image, a region of bone-tissue interface, and a homogeneous brain tissue region, respectively.
Download figure:
Standard image High-resolution image2.2. Comparison of personalized and reference phantoms
The second part of this study was to determine if a personalized, 3D printed phantom is superior for assessing patient-specific electron beam radiotherapy plans. This was accomplished by designing and printing a personalized head phantom based on patient CT image data, then comparing the dose distributions calculated in the personalized phantom, an adult male reference phantom (Atom Model 701, CIRS, Norfolk VA), and the patient. The planned dose distribution in the patient served as the best estimate of the true dose distributions (figure 3).
Figure 3. An illustration of the advantages of personalized phantoms for patient-specific radiation dose measurements. The patient's nose was surgically amputated prior to radiation therapy. The red arrows indicate the beam direction, the red line is the isodose distribution, the blue object is the bolus, and the green sphere is the treatment volume. The center (reference) and right (personalized) graphics show how the two phantoms differ in modeling the dose distributions in the patient (left).
Download figure:
Standard image High-resolution imageTo design the personalized phantom, a CT image set of a patient's head with a partial nose amputation was selected as the basis for design. The CT image set was imported into the TPS, where the relevant anatomy was contoured. Due to printer limitations, the dynamic range of mass densities of soft tissues and bone could not be continuously replicated in the printed phantoms. (It was not possible to reliably achieve the desired printed mass density based on the printing quantity of infill density.) To eliminate density variations from this study altogether, we reassigned CT numbers in all images to correspond to either unit density tissue or air. Binarization of the CT data used locally drawn ROIs and tissue thresholding. Voxels with an average CT number greater than 900 HU were designated as plastic. The resulting phantom contours are shown in figure 4(a). The contours were exported, converted to STL files, and printed following the same procedure described in section 3.1. The dimensions of the printed phantom (figure 4(b)) were measured at selected locations with a caliper and compared with corresponding dimensions using software tools to measure bony landmarks in the CT scan. Measurements agreed within 2 mm.
Figure 4. Upper: Transverse (A) and sagittal (B) views of the patient anatomy and printed phantom design at isocenter. The orange line represents the phantom exterior surface. The printed phantom design includes the (1) ethmoidal sinus, (2) sphenoidal sinus, and (3) oral cavity. Lower: Photograph of the corresponding 3D printed phantom in frontal view, revealing nose amputation and nasal cavity.
Download figure:
Standard image High-resolution imageTo assess the dosimetric accuracy achieved with the personalized and reference phantoms, dose distributions from electron beams were calculated with the TPS (which was validated previously and in section 3.1). A simulated 6 × 6 cm2 electron beam was positioned on the patient CT, perpendicular to the patient's nasopharyngeal region for these calculations (figure 5). Doses were calculated at 6, 9, 12, 16, and 20 MeV nominal electron beam energies. The personalized and reference phantoms were imaged using the previously mentioned CT scanner and the image sets were imported into the TPS. Each image set was registered to the patient CT data, and the treatment fields were copied to each phantom.
Figure 5. A sagittal view of the dose calculations for a 12 MeV electron beam incident on the patient. The isodose lines show the relative dose distributions in the patient for the irradiation simulation. (A) indicates the region where the nose was amputated, (B) is the upper oral cavity, (C) is the ethmoidal sinus, and (D) is the sphenoidal sinus.
Download figure:
Standard image High-resolution imageThe dose distribution calculated in the patient was compared to those in the reference and personalized phantoms. We compared the dose distributions using commercial quality assurance software (SNC Patient, Sun Nuclear Corporation, Melbourne, FL). For each phantom, the patient and phantom dose distributions were registered together. The dose distributions were compared using gamma index analysis criteria of 3% DD and 3 mm DTA, and a dose threshold of 10%.
3. Results
3.1. Comparison of printed and molded anthropomorphic phantom slices
We verified that the geometric properties of a molded anthropomorphic phantom were replicated with a 3D printed phantom. The radiometric properties of the phantoms were similar but not identical because we lacked a capability to print bone-substitute material. Table 1 lists gamma index analysis pass rates at all electron beam energies for three ROIs shown in figure 2. The pass rates were generally higher at 12 MeV and higher beam energies. At 6 MeV, a lower pass rate was expected because the water equivalent thickness of the slices is greater than the range of the beam in water. Figure 6 plots the gamma index failure locations for 12 MeV electrons. The impact of the bone heterogeneities on the absorbed dose distribution is readily apparent. These failures are largely caused by differences in multiple coulomb scattering (MCS) of electrons, where the differences in MCS caused by the density variations in the molded slice that were not present in the printed slice. In contrast, the homogenous brain region in ROI 3 shows a low failure rate, indicating that the printed and molded slices provide similar dosimetric in that region of soft tissue.
Table 1. Gamma index analysis pass rates for the different regions of interest (ROI) used in comparing the exit doses for the molded and printed phantom slices. The ROIs are shown in figure 3.
| Electron beam energy (MeV) | Gamma index pass rate (%) | ||
|---|---|---|---|
| ROI 1 | ROI 2 | ROI 3 | |
| 6 | 11.4 | 1.9 | 50.4 |
| 9 | 45.9 | 41.3 | 99.0 |
| 12 | 78.9 | 64.0 | 93.5 |
| 16 | 87.5 | 79.7 | 99.8 |
| 20 | 77.1 | 66.4 | 99.3 |
Figure 6. Graphic of the full image gamma index map for the printed and molded anthropomorphic slice irradiations. The irradiations were performed using a therapeutic 12 MeV electron beam. The red regions are failing points and the greyscale regions are passing points. The ROIs are indicated by the white boxes.
Download figure:
Standard image High-resolution image3.2. Comparison of personalized and reference phantoms
To compare the performance of personalized and reference phantoms, we designed and printed a personalized head phantom based on patient CT image data. Dose distributions calculated on images of the personalized and reference phantoms were then compared against those on the patient. The latter served as the best estimate of the true dose distributions (i.e. good performance of the phantom was demonstrated by obtaining good agreement between the dose distribution in a phantom and the patient).
Table 2 lists the gamma index pass rates for the comparisons of results from dose calculations on CT images of the phantoms against those from the images of the patient. At all electron beam energies considered, the dosimetry performance of the personalized phantom was superior to that of the reference phantom. The goal of 60% or better pass rate was met at 16 and 20 MeV but not at lower energies. The difficulty of charged particle dosimetry increases with decreasing beam energy, and the lower pass rates below 12 MeV are not entirely unexpected, especially given the current limitations on modulating printed mass density and printing only one material (PLA). Consequently, we next digress briefly regarding possible explanations for the lower pass rates at lower beam energies.
Table 2. Gamma index analysis pass rates for the dosimetric comparison of the head phantoms to the patient at various nominal electron beam energies.
| Electron beam energy (MeV) | Gamma index pass rate (%) | |
|---|---|---|
| Personalized printed phantom | Reference molded phantom | |
| 6 | 40.7 | 27.3 |
| 9 | 43.7 | 25.8 |
| 12 | 53.4 | 28.1 |
| 16 | 63.3 | 32.8 |
| 20 | 69.7 | 41.3 |
Recall that we suppressed differences in observed differences in dose distributions between the printed personalized phantom and the patient by manually reassigning or overriding tissue densities in the CT images of the patient and phantoms. This was beneficial in it suppressed a source of experimental bias and it also facilitated a more direct comparison of results. However, to improve pass rates, a better long-term approach would be to develop printing capabilities that allowed continuous and calibrated modulation of mass density, a wider dynamic range of mass density (especially high densities needed for bone), and to print with multiple materials (e.g. soft tissue and bone substitute plastics). That said, the present results revealed that, even with the current limitations on printing capabilities, the superior performance of the printed personalized phantom over the molded reference phantom was still evident. In fact, this superiority underscores the importance to accurately model surface geometry (e.g. the amputated nose) and pronounced internal heterogeneities (e.g. the nasal sinus and paranasal sinus cavities and their interfaces) (figure 7).
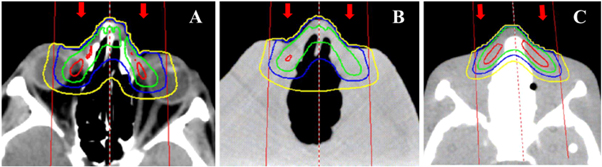
Figure 7. Transverse images of the dose distributions from 6 MeV electron beam in (A) the patient, (B) the printed phantom, and (C) the reference phantom. The red, blue, green, and yellow isodose lines correspond to 80%, 60%, 40%, and 20% of the maximum dose. The beam placement is shown by the red lines and the beam direction is indicated by the red arrows.
Download figure:
Standard image High-resolution image4. Discussion
This study demonstrated that personalized 3D printed phantoms are feasible for research applications requiring radiation dose measurements. Furthermore, the study revealed that for non-reference anatomy, the personalized 3D printed phantom provided superior performance for electron beam treatments. We achieved gamma pass rates of greater than 60% at 16 and 20 MeV electron beam energies.
The implication of this study is that personalized 3D printed phantoms appear to be well suited for dosimetry research in patient anatomy that differs substantially from reference anatomy. Example applications include phantoms that can accommodate prostheses or other implants that would enable measurements of their dosimetric impact. In turn, such measurements could inform future refinements to treatment techniques and protocols. These finding are qualitatively coherent with the findings of previous studies, such as those from Homolka et al (2017) on applications to therapy and of Filippou and Tsoumpas (2018) on application to imaging.
To the best of our knowledge, this was the first study to examine the feasibility of 3D printed phantoms that model both internal and external patient geometry for use in electron beam dosimetry. Furthermore, we sampled a broad range of clinically relevant electron energies. A related study, using the same electron beam energies, proved the concept of fabricating a patient-specific bolus for electron beam therapy (Su et al 2014). They reported improvements in patient dosimetry and the sparing of adjacent healthy tissue.
Two limitations of this study pertain to the limited realism of the printed phantoms. Specifically, due to technical limitations of our system, we were not able to 3D print with continuously varying mass density. Other studies in printing modalities have shown improvements in phantom imaging applications through different printing methods compared to the standard infill modality used in this study (Tino et al 2019b). Furthermore, our PLA printing material did not mimic the higher mass density and different elemental composition of bone. However, these are not serious limitations because they are being addressed in other studies. Price et al (2020) for example, reported using acrylonitrile butadiene styrene plastic with CaTiO3 powder as bone equivalent material. They reported the attenuation properties were similar to reference bone material.
Even with the aforementioned limitations of the methods used in this study, the approach of personalized 3D printed phantoms yielded uniformly superior results compared to the reference phantom. This underscores that, for subjects with unique anatomy, it is important for phantoms to have realistic external contours and to include internal air cavities, both of which are readily achievable with currently available 3D printing methods. A similar study by Craft and Howell (2017) focusing on a postmastectomy torso phantom confirms these findings, as well as showing that the technology is applicable to other body regions for better modeling unique patient anatomy.
5. Conclusions
3D printed personalized anthropomorphic phantoms are feasible for radiation dose measurements and provide improved dosimetric realism and accuracy. Further improvements in the phantom design and printing processes are called for, including printing with precise and continuous control over mass density and additional tissue equivalent materials, especially for bone.
Acknowledgments
We thank Vincent Celluci and Warren Hull of Louisiana State University for their discussion and guidance in the 3D printing portions of this work, and Paul Maggi and Joseph Steiner for helpful discussions. We thank Michelle Lis for assistance in the preparation of this manuscript. This work was supported in part by the US Nuclear Regulator Commission (grant NRC-HQ-84-15-G-0017) and the Bella Bowman Foundation.