Abstract
Facile synthesis of core–shell magnetic MOFs for drug delivery is of significance due to the advantages of high drug load and easy separation. In this work, magnetic metal organic frameworks (MOFs, Fe3O4-NH2@MIL101-NH2) core–shell nanoparticles were synthesized rapidly in water phase by microwave irradiation using Fe3+ and 2-amino-1,4-benzenedicarboxylate (BDC-NH2) as metal ions and ligands respectively. The resulting magnetic MOFs exhibit large surface areas (96.04 m2 g−1), excellent magnetic response (20.47 emu g−1) and large mesopore volume (22.07 cm3 g−1) along with spherical morphologies with the diameters ranging from 140–330 nm. Using doxorubicin (DOX) as a model drug, the drug loading capacity of Fe3O4-NH2@MIL101-NH2 could reach 36.02%, substantially higher than pristine MIL101-NH2. Importantly, the release of DOX could be controlled by pH as well as the meso pore size of MOFs. The cytotoxicity assay showed that the magnetic MOFs have low cytotoxicity and good biocompatibility. The results suggest great potential of the magnetic MOFs core–shell nanoparticles fabricated in this study on controlled drug release of DOX.
Export citation and abstract BibTeX RIS
1. Introduction
Metal-organic frameworks (MOFs), an emerging class of organic–inorganic hybrid porous materials built from metal ions and organic linkers [1], are also called porous coordination polymers or coordination polymer networks [2]. Due to its special coordination structure, its structure can be designed according to targeted properties based on the geometries of the organic building blocks and coordination mode of the inorganic metal ions [3]. Thus, MOFs possess well-defined pores and large internal surface areas. Another key feature of MOFs is easy functionalization and modification to the surface of the inner pore [2]. The works of other researchers presented that the MOFs composites have excellent adsorbent properties, good biodegradation and low toxicity [4–6]. These special physical–chemical properties make them leading candidates to gas and small molecule adsorption [7], ion exchange [8], heterogeneous catalysis [9], photo-chemical [10], drug delivery [5, 11–13] and biomedical imaging fields [5, 12, 13].
Incorporating magnetic materials within MOFs is an important and effective way to achieve unique functions originating from the synergistic effect of magnetic MOFs for multifunctional applications [3]. Magnetic materials, such as ferromagnetic metal oxide NPs (e.g. Co, Ni and Fe) and superparamagnetic metal oxide NPs (e.g. Co3O4, γ-Fe2O3 and Fe3O4) [3], with the advantages of excellent biocompatibility [14], rapid separation efficiency [15] and high magnetic responsiveness [11], are prevalent in MR imaging which could significantly shorten transverse relaxation time [14]. Therefore, magnetic MOFs composite appears of extraordinary interest as the composite can carry and deliver specific drugs in biological systems as well as easy separation and quick purification. The synergy between MOFs and magnetic nanoparticles in the composite showed sustained release with no burst effect, high drug loading capacity meanwhile high transverse relaxivity for MR imaging with good biocompatibility and low toxicity [4, 6, 16].
Till now, reported methodologies to fabricate magnetic MOFs are usually using organic solvent (e.g. dimethylformamide (DMF), diethylformamide, ethanol, methanol) to dissolve organic ligands participating the MOFs reaction [5]. The MOFs formation usually needs several hours (>30 min) under high temperature (>70 °C) with a time-consuming layer-by-layer cycle [16–18]. For instance, Fu et al used DMF solution to dissolve MOFs precursors and reacted at 80 °C for 12 h, followed by incubating at 100 °C for 24 h to obtain Fe3O4@UiO-66 core–shell composites [4]. Chen et al repeated a cycle number of 16 to obtain a shell with ideal thickness of Fe3O4@C@MIL-100(Fe) core–shell composites [6]. Other reports generated magnetic nanoparticles immobilized in the pore or the skeleton of the MOFs, which also needed long time, high temperature and high energy consumption [19, 20], e.g. Kun-Yi Andrew Lin carbonized the precursor in N2 atmosphere at 600 °C for 6 h to obtain the magnetic core [20]. Therefore, developing facile method for magnetic MOFs preparation is urgently needed for time-saving and avoiding use of organic solvents.
In this work, MIL 101 coated Fe3O4 magnetic MOFs were synthesized in water phase within several minutes under microwave irradiation, as shown in scheme 1. Fe3O4–NH2 nanoparticles with an amino group modified surface, synthesized through the solvothermal method [21], could be easily connected with carboxylic groups of the organic ligand. MIL101-NH2 was a kind of metal organic frameworks classified by Materials of Institute Lavoisier with a huge pore 29–34 Å [5], suitable for majority kinds of drug adsorption. Thus, we enlist Fe3O4-NH2 nanoparticle as the magnetic core, Fe3+ and 2-amino-1,4-benzenedicarboxylate (BDC–NH2) as the metal ions and ligands dispersed in water to prepare MIL101-NH2 (shell). The whole process reacted without high energy consumption, pressure, toxic-solvent or toxic-gas. The final product could be collected by an adscititious magnet. The Fe3O4–NH2@MIL101-NH2 nanoparticles were characterized by Fourier transform infrared (FT-IR), X-ray powder diffraction (XRD), BET, SEM, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and VSM. DOX, a typical water-soluble anti-cancer drug, was selected as a model to test the drug loading capacity of the magnetic MOFs by an adsorption method. The synthesis conditions of the magnetic MOFs composite (such as reagent molar ratio and reaction time) were optimized for high drug loading capacity. The release performance of DOX has obvious responsiveness to pH and molar ratio of Fe3O4–NH2 to MIL101-NH2. Our results demonstrate the efficient loading and controlled release of DOX from magnetic core–shell MOFs Fe3O4-NH2@MIL101-NH2, which can be synthesized in minutes in water phase.
Scheme 1. Schematic of the fabrication of magnetic MOFs for DOX loading and controlled release.
Download figure:
Standard image High-resolution image2. Materials and method
2.1. Materials
Ferric chloride, Ferric chloride hexahydrate, Ethylene glycol (EG), Sodium acetate, Ethylene diamine and Dimethyl formamide (DMF) were purchased from Sinopharm Chemical Reagent Co., Ltd 2-Aminoterephthalic acid was purchased from Shanghai Cablebridge biotechnology Co., Ltd Doxorubicin hydrochloride (DOX) was purchased from Wuhan Yuancheng Technology Co., Ltd. Other reagents and materials used were commercially available without any purification. The water used in the experiments was 18.2 MΩ cm−1 ultrapure water.
2.2. Preparation of Fe3O4-NH2
Fe3O4 nanoparticles were prepared according to the method reported by Peng et al [21]. 2.03 g FeCl3, 3.00 g NaAc and 12.0 ml Ethylene diamine were dissolved in 50.0 ml EG at 70 °C to form a brown solution. The mixed solution was transferred into a 100 ml teflon-lined stainless-steel reaction kettle and maintained at 205 °C for 10 h. Then, the solution was cooled to room temperature. Later, the black magnetic nanoparticles were collected by a magnet and washed several times by water to remove salt. After that, the products were dispersed in a constant volume of water for further use.
2.3. Preparation of Magnetic MOFs
The MOFs was prepared according to the method reported by Patricia Horcajada [5] with some modifications. Specifically, 0.13 g FeCl3 · 6H2O (0.5 mmol) was dissolved in 50 ml water, and 0.09 g 2-aminoterephthalic acid (0.5 mmol, BDC-NH2) was dispersed in 50 ml water by ultrasonic for 5 min. Then, Fe3O4 suspension solution, FeCl3 solution and BDC-NH2 dispersion solution were mixed in a round-bottom flask and ultrasound for another 5 min. The mixture was heated under microwave irradiation at 400 W for several minutes. The obtained magnetic MOFs was recovered by a magnet and washed with water for several times.
Pure MIL101-NH2 was synthesized using a similar method with the absence of Fe3O4. MIL101-NH2 was collected by centrifugation at 16 000 rpm for 15 min. The solid was washed with absolute DMF for whole night to remove the free acid. Later, the solid was vacuum-dried overnight at 60 °C and stored in a desiccator.
2.4. Loading of DOX on magnetic MOFs
Magnetic MOFs (40 mg) and 25.0 ml DOX solutions with different concentrations were added into 50 ml glass conical flask and then shaken at 90 rpm for 24 h under 30 °C ± 0.5 °C. The DOX loaded composite were separated and collected by the aid of an adscititious magnet and freeze-dried overnight.
The amount of DOX loaded on the magnetic MOFs was estimated by measuring the concentration of DOX before and after adsorption, which was quantitated by measuring the absorbance of DOX at 480 nm with a UV spectrophotometer. The drug loading capacity-D (mg g−1) was calculated according to the following equation:
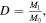
where M0 is the quantity of composite including M1 and MMOFs (g), M1 is the quantity of DOX (mg) contained by the composite.
2.5. Drug release
DOX released from the magnetic MOFs composite was analyzed in different pH buffer solutions. The analysis experiment was taken in pH 7.4 PBS buffer if there were no special instructions. Typically, 10 mg composite were added into 25 ml buffer solution at controlled temperature and gently agitated. At predetermined intervals, the magnetic MOFs were separated using an adscititious magnet and 4 ml of the supernatants was withdrawn from the system. 4 ml fresh buffer solution was added into the system to maintain the total volume. The absorbance of the supernatants was monitored at 480 nm with a UV spectrophotometer. The concentration of DOX was calculated from the calibration curve obtained with known amounts of DOX concentrations. Each assay was performed in triplicate and reported as the average value.
2.6. Cell cytotoxicity and IC50 assay of the magnetic MOFs
The Hela cell lines were provided by the China Center for Type Culture Collection. The Hela cells were cultured in DMEM supplemented with 10% Fetal Bovine Serum at 37 °C and 5% CO2.
In vitro cytotoxicity was measured by performing protocol of cell counting kit-8 (CCK-8) assay on the Hela cells. Cells were seeded into a 96-well cell culture plate at 5 × 103 per well, and were cultured at 37 °C and 5% CO2 for 24 h; different concentrations of magnetic MOFs (0, 5, 10, 25, 50 μg ml−1), DOX (0, 1, 2, 5, 10 μg ml−1) and DOX loaded magnetic MOFs (with equivalent DOX concentrations 0, 1, 2, 5, 10 μg ml−1) diluted in DMEM were then added to each well. The cells were subsequently incubated for 24 h at 37 °C under 5% CO2. Untreated cells servsed as 100% cell viability. Thereafter, 10 μl CCK-8 was added to each well and the plate was incubated for an additional 4 h at 37 °C under 5% CO2. The optical density OD value (Abs.) of each well, was measured by means of a Tecan Spark TM 10M monochromator-based multifunction microplate reader at 450 nm. The following formula was used to calculate the inhibition of cell growth:
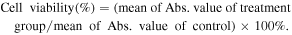
2.7. Characterization
Morphological features and surface characteristics of samples were investigated by taking photographs using scanning electron microscopy (Zeiss, Germany) and transmission electron microscopy (Hitachi H-7000FA, Japan). Element mapping of the MOFs was conducted on HAADF-STEM. X-ray powder diffraction (XRD) patterns of samples were recorded on a PAN-alytical X' Pert Pro x-ray diffractometer with a Cu Ka radiation at a scanning rate of 0.02° s−1 from 4° to 80°. Samples were ground to powders in order to eliminate the influence of crystalline orientation. The patterns of the magnetic hysteresis loops of the samples were characterized at room temperature using a HH-15 model vibrating sample magnetometer. The absorbance of the samples was monitored using UV-2550 spectrophotometer (Shimadzu, Japan). FT-IR spectra of the samples were recorded on a Nicolet 5700 FT-IR spectrometer. The specific surface area of the samples were measured at 77 K through N2 sorption analysis using the BET method on a Belsorp-mini Ⅱ (Japan).
3. Results and discussion
3.1. Synthesis and characterization of magnetic MOFs nanoparticles
In a typical process, Fe3O4-NH2 nanoparticles were directly dispersed into the synthetic precursor of MIL101-NH2 composed of FeCl3 · 6H2O , BDC–NH2 and H2O, and the reaction was accomplished through a simple microwave-heated procedure. This method avoids time-consuming layer-by-layer MOF growth [16] and further modification of core particles, which is much simpler than most of the previous methods [4] of synthesizing Fe3O4 nanoparticle-based core–shell composites.
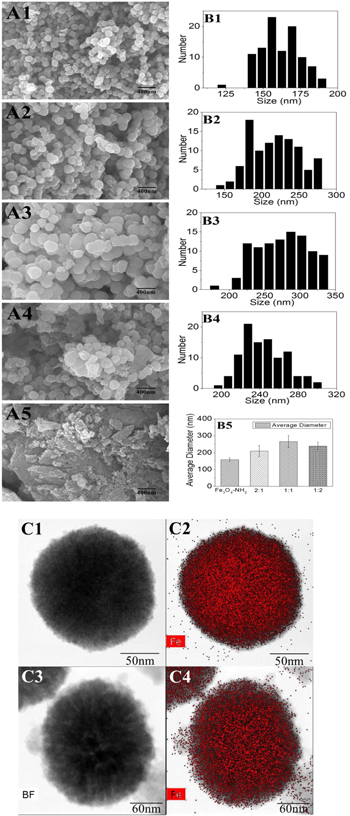
As figure 1(A1)–(A4) shown, the magnetic MOFs composite presents a spherical morphology with the diameters ranging from 140–330 nm. The pure MIL101-NH2 shows a type of amorphous form. The diameter of the magnetic MOFs was obviously increased due to the coating of MIL101-NH2 layer. The average diameter of Fe3O4-NH2 nanoparticles was only 158 nm, while the diameter was increased to 268 nm at the molar ratio of 1:1, demonstrating the covering of MIL101-NH2 on the surface of the Fe3O4-NH2 nanoparticles. Figure 1(B) showed the statistical analysis of diameter of the nanoparticles with different molar ratio of Fe3O4-NH2 to MIL101-NH2. The optimal condition to form core–shell structured magnetic MOFs was the molar ratio of Fe3O4-NH2 to MIL101-NH2 as 1:1, resulting little visible amorphous MIL101-NH2 and thick MOFs covering layer.
Figure 1. A. SEM imaging of magnetic MOFs composite. (1–5 corresponding to Fe3O4-NH2, Fe3O4-NH2@MIL101-NH2(2:1), Fe3O4-NH2@MIL101-NH2 (1:1), Fe3O4-NH2@MIL101-NH2 (1:2), MIL101-NH2) B. Statistics of the average diameters. (1–4 corresponding to Fe3O4-NH2, Fe3O4-NH2@MIL101-NH2(2:1), Fe3O4-NH2@MIL101-NH2 (1:1), Fe3O4-NH2@MIL101-NH2 (1:2) and B5. average diameter.) C. TEM imaging and element mapping of Fe3O4-NH2 (C1, C2) and Fe3O4-NH2@MIL101-NH2 (1:1) (C3, C4).
Download figure:
Standard image High-resolution imageFurthermore, HAADF-STEM and elemental mapping analysis were used to verify the core–shell structure of the Fe3O4-NH2@MIL101-NH2 composite. As figure 1(C) showed, a clear contrast between the core and shell was obtained wherein the core appeared dark, whereas the shell appeared lighter. Moreover, the elemental mapping analysis revealed that Fe elements were distributed in the core and shell. Comparing magnetic MOFs in C4 with Fe3O4-NH2 in C2, the distribution of Fe element was much sparse at the edge of the MIL101-NH2 shell. All the results demonstrated the successful formation of a core–shell structure in the Fe3O4-NH2@MIL101-NH2 composite.
In addition, XRD was also employed to demonstrate the success synthesis of magnetic MOFs from 4° to 80° (figure 2(A)). Fe3O4 nanoparticles, synthesized through a solvothermal method, were spherical with a mean diameter of 158 nm and in the cubic phase (JCPDS: 19-0629) [4, 21]. The characteristic peaks of Fe3O4-NH2 nanocrystal at 30.2°, 35.5°, 43.2°, 57.3° and 62.8° were also presented in the patterns of the Fe3O4-NH2@MIL101-NH2 composite, indicating that the core–shell Fe3O4-NH2@MIL101-NH2 composites were synthesized by an in situ self-assembly of MIL101-NH2 on the surface of Fe3O4-NH2 nanoparticles. The characteristic peak at 8.5° was not so prominent corresponded to the amorphous MIL101-NH2 observed by the SEM and TEM images [13].
Figure 2. (A) XRD patterns of magnetic MOFs composite. (B) Magnetization hysteresis curves of magnetic MOFs. (Green, red, blue and black curves corresponding to Fe3O4-NH2, Fe3O4-NH2@MIL101-NH2 (2:1), Fe3O4-NH2@MIL 101-NH2 (1:1) and Fe3O4-NH2@MIL101-NH2 (1:2) respectively). (C) FT-IR spectra of magnetic MOFs. (D) BET isotherms of magnetic MOFs.
Download figure:
Standard image High-resolution imageSuperparamagnetism is essential to MR imaging [14]. Thus, the magnetic properties of the Fe3O4-NH2@MIL101-NH2 composites were investigated in a magnetic field range from −10 000 to +10 000 Oe at room temperature (figure 2(B)). The saturation magnetizations (Ms) of the Fe3O4-NH2@MIL101-NH2(1:1) and Fe3O4-NH2@MIL 101-NH2(2:1) were 20.47 and 21.32 emu g−1, which were smaller than that of Fe3O4-NH2 nanoparticles (27.67 emu g−1) due to the encapsulation by the MIL101-NH2 layer. Due to the inclusion of amorphous MIL101-NH2, the Ms of Fe3O4-NH2@MIL101-NH2(1:2) was only 3.04 emu g−1. The high Ms value and the existing hysteresis loop without significant coercivity and remanence in the magnetization hysteresis curves demonstrated the strong superparamagnetic character of the as-synthesized Fe3O4-NH2@MIL101-NH2 composites [16].
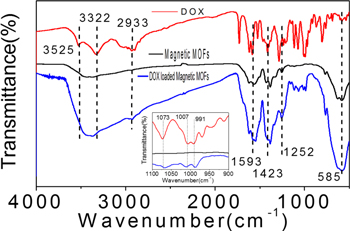
In figure 2(C), the characteristic peaks of MIL101-NH2 at 1593 cm−1, 1498 cm−1, and 1423 cm−1 and Fe3O4-NH2 at 585 cm−1 in the FT-IR spectrum of Fe3O4-NH2@MIL101-NH2 indicated the formation of the Fe3O4-NH2@MIL101-NH2 composites [6, 13, 21]. The two peaks at 3525 and 3382 cm−1 in the spectrum of MIL101-NH2, belonged to the asymmetric and symmetric stretching absorptions of the primary amine groups in the BDC-NH2 ligands and Fe3O4-NH2. The peak at 760 cm−1 indicated the symmetric stretching of C–N bond of the BDC-NH2 [22], The FT-IR results further verified the successful growth of the MIL101-NH2 shell on the Fe3O4-NH2 core.
Taking Fe3O4-NH2@MIL101-NH2 (1:1) as an example, the porosity of the magnetic MOFs composite was evaluated by nitrogen adsorption-desorption measurements. The composite shows a typical IV type isotherm with H3 hysteresis loop (figure 2(D)) [6]. The BET surface area of the sample was 96.04 m2 g−1 with the average pore size 6.68 nm (figure S1(A) is available online at stacks.iop.org/NANO/28/495601/mmedia), which was obviously increased in contrast to 2.96 nm of MIL101-NH2 (figure S1(B)) [5].
3.2. Drug loading/release of magnetic MOFs nanoparticles
DOX was used as a model drug to test the drug loading capacity of the magnetic MOFs. DOX was loaded to the magnetic MOFs by adsorption and the product was analyzed by FT-IR, as figure 3 shown. The characteristic peaks of magnetic MOFs at 1593, 1423, and 1252 cm−1 and Fe3O4-NH2 at 585 cm−1 were remained in the spectrum of DOX loaded MOFs. The characteristic peaks of DOX at 2933, 1071, 1007 and 991 cm−1 also appeared in the spectrum of DOX loaded magnetic MOFs, indicating the incorporation of DOX molecules in DOX loaded magnetic MOFs composite [4, 23].
Figure 3. FT-IR spectra of DOX, magnetic MOFs and DOX loaded magnetic MOFs.
Download figure:
Standard image High-resolution imageThe DOX loading capacity was largely affected by the composite ratio of Fe3O4-NH2 to MIL101-NH2. As shown in table 1, The DOX loading capacity of pure Fe3O4-NH2 nanoparticles was 9.46 mg g−1 and the loading capacity of Fe3O4-NH2@MIL101-NH2 was substantially improved to over 39 mg g−1. However, the DOX loading capacity of pure MIL101-NH2 was only 5.29 mg g−1, which was probably because that the pore size of the MIL101-NH2 was small and not suitable for the DOX adsorption [5]. As the results of the porosity listed in table 1, the mean pore diameter of pure MIL101-NH2 synthesized by this method was only 2.96 nm, while the pore diameter of magnetic MOFs composite were more than 6 nm. According to previous report, the pore size had significant effect on the adsorption property of the MOFs [24, 25]. Although the average pore size of Fe3O4-NH2 nanoparticles was large (14.38 nm), the BET surface area-S was only 34.56 m2 g−1. The high drug loading capacity of Fe3O4-NH2@MIL101-NH2 composites was probably attributed to the large surface area of the MIL101-NH2 shell and suitable pore size for the entrapment of DOX in MIL101-NH2 [4].
Table 1. The porosity analysis of magnetic MOFs with different molar ratio by the BET method.
| Samples (molar ratio) | DOX loading capacity (mg g−1) | Vm (cm3 g−1) | S (m2 g−1) | Mean pore diameter (nm) |
|---|---|---|---|---|
| Fe3O4-NH2 | 9.46 ± 0.18 | 7.94 | 34.56 | 14.38 |
| Fe3O4-NH2@MIL101-NH2(2:1) | 39.79 ± 1.99 | 10.01 | 43.59 | 8.94 |
| Fe3O4-NH2@MIL101-NH2(1:1) | 48.77 ± 3.48 | 22.07 | 96.04 | 6.68 |
| Fe3O4-NH2@MIL101-NH2(1:2) | 44.88 ± 2.24 | 19.69 | 85.71 | 6.02 |
| MIL101-NH2 | 5.29 ± 0.13 | 37.19 | 161.88 | 2.96 |
Next, we showed the microwave time also affected the DOX loading capacity on magnetic MOFs. Table 2 showed the tendency of the specific surface area-S and the total pore volume–Vm of magnetic MOFs (Fe3O4-NH2@MIL101-NH2 1:1) changing with reaction time. In the beginning of 5 min, S and Vm increased with the reaction time. Then, they both decreased with the time extended. The DOX loading capacity of the magnetic MOFs correlated well with S and Vm. Thus, the magnetic MOFs microwaved for 5 min had the largest BET surface area 96.04 m2 g−1 and total pore volume 22.07 cm3 g−1, resulting an optimum DOX loading capacity of 48.77 mg g−1. As large BET surface area could provide more chemical binding site and pore volume, as a result contribute to the DOX chemical adsorption and physical entrapment [26]. Further increase of the drug load could be accomplished by increasing the concentration of DOX. As the concentration of DOX increased from 200 to 10 000 mg l−1, the drug loading capacity increased from 48.77 to 360.16 mg g−1 (figure S2), which is among the optimal results that reported previously [5, 27].
Table 2. The porosity analysis of magnetic MOFs with different microwave time by the BET method.
| Microwave time | DOX loading capacity (mg g−1) | Vm (cm3 g−1) | S (m2 g−1) | Mean pore diameter (nm) |
|---|---|---|---|---|
| 3 min | 25.50 ± 1.81 | 5.28 | 22.99 | 4.72 |
| 5 min | 48.77 ± 3.46 | 22.07 | 96.04 | 6.68 |
| 10 min | 40.17 ± 2.85 | 20.11 | 87.52 | 5.11 |
| 15 min | 34.75 ± 2.47 | 15.03 | 65.42 | 6.11 |
| 20 min | 12.35 ± 0.88 | 2.91 | 12.65 | 16.73 |
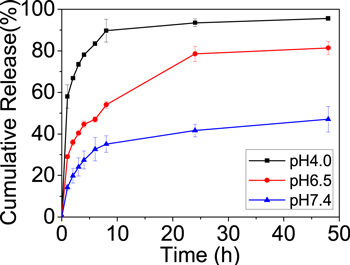
The release behavior of DOX from the magnetic Fe3O4-NH2@MIL101-NH2 composite differs from Fe3O4-NH2 nanoparticles and pristine MIL101-NH2. As shown in figure 4, Fast release of DOX was observed from Fe3O4-NH2 nanoparticles and pristine MIL101-NH2. On the contrary, the release of DOX from Fe3O4-NH2@MIL101-NH2 was relatively slow and only 37%–61% of DOX was released in 48 h, suggesting the coating of MOFs layer could effectively reduce the DOX burst release of the Fe3O4-NH2 nanoparticles. Moreover, the release of DOX was affected by the ratio of Fe3O4-NH2 to MIL101-NH2. It was reported that large pore sizes could reduce the steric diffusion resistance [26, 28]. The average mesopore size of Fe3O4-NH2@MIL101-NH2 decreased from 8.94 to 6.02 nm with the increased ratio of MOFs, leading to slower DOX release.
Figure 4. Cumulative release profiles of DOX from magnetic Fe3O4-NH2@MIL101-NH2 composite. The release was performed in pH 7.4 PBS at 30 °C, the data points are presented as the means ± standard deviations (n = 3).
Download figure:
Standard image High-resolution imageThe stability of the nanocarrier in a wide pH range from basic to acidic is essential for drug loading/release. Thus, we investigated the controlled release of the as-synthesized magnetic Fe3O4-NH2@MIL101-NH2 composites at different pH values (4.0, 6.5, 7.4). Taking the magnetic MOFs composite-Fe3O4-NH2@MIL101-NH2 (1:1) as an example, the DOX release curves under various pHs were shown in figure 5. In contrast to the slow and partial release of DOX from the magnetic MOFs composite in simulated body fluid at pH 7.4, 53.9% and 89.7% of the loaded DOX were released in 8 h when the composite was incubated in simulated tumor cell microenvironment (pH 6.5) [29] and weak acidic environment (pH 4.0). This was caused by the decomposition of MIL101-NH2 in acid buffer due to the protonation of BDC-NH2 [5]. The results in figure 5 indicated that the release of DOX from Fe3O4-NH2@MIL101-NH2 could be adjusted by varying pHs, favoring the targeting of tumor cells and on site DOX release.
Figure 5. Cumulative release profiles of DOX from magnetic Fe3O4-NH2@MIL101-NH2 composite under different pH conditions at 30 °C. The data points are presented as the means ± standard deviations (n = 3).
Download figure:
Standard image High-resolution image3.3. Cytotoxicity of magnetic MOFs nanoparticles
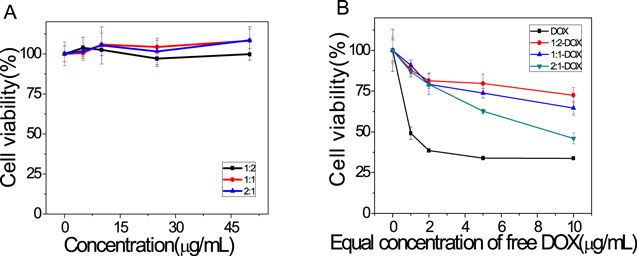
As a potential drug carrier that would be applied in vivo system, the cytotoxicity of magnetic MOFs is of great importance for its bioapplication. As the model drug-DOX was an antitumor antibiotic, we use Hela cell lines to investigate the cytotoxicity of magnetic MOFs. The result in figure 6(A) showed the magnetic MOFs co-incubated with Hela cells for 24 h exhibited little cytotoxicity in the analyzed concentration range (0–50 μg ml−1). DOX loaded magnetic MOFs with equivalent dose of DOX exhibited similar cytotoxic effect as the DOX upon Hela cells in a dose-dependent manner (figure 6(B)). Half of the Hela cells died (IC50) by the chemotherapeutic effect of DOX co-incubated for 24 h at 37 °C, 5% CO2 when the DOX concentration was above 1 μg ml−1. The cell viability of the DOX loaded magnetic MOFs were 72.52%, 64.62% and 45.99% for 1:2, 1:1 and 2:1 magnetic MOFs samples (molar ratio Fe3O4-NH2 to MIL101-NH2) at the equivalent DOX concentration 10 μg ml−1, which has a good correspondence with the drug release profiles in figure 4. In contrast to free DOX, DOX loaded magnetic MOFs show weak cytotoxicity due to the slow release of DOX in cell culture medium. All these results were consistent with the previous reports [30]. Hence, the results showed that the magnetic MOFs with low cytotoxicity and high biocompatibility is suitable for drug delivery.
Figure 6. Hela cell viabilities values (%) estimated by CCK-8 method after the co-incubation with magnetic MOFs (A), free DOX and DOX loaded magnetic MOFs (B) for 24 h. The data points are presented as the means ± standard deviations (n = 6).
Download figure:
Standard image High-resolution image4. Conclusion
In this study, magnetic MOFs Fe3O4-NH2@MIL101-NH2 nanoparticles were prepared through a rapid and facile green route. The particle size ranging from 140–330 nm could be adjusted by the synthesize conditions. The cover layer of MIL101-NH2 can elevate the drug loading capacity of the composite using DOX as a model. The mean pore diameter of MIL101-NH2 influenced the loading capacity and release behavior of DOX. In addition, the release of DOX was accelerated in weak acid environment, facilitating the targeting drug release to tumor cells. The composite with good superparamagnetism and low cytotoxicity could be used as a potential target drug delivery platform.
Acknowledgments
The work was financially supported by the Natural Science Foundation of the Hubei province, China (No. 2014CFB728), Fundamental Research Funds for the Central Universities (2042016kf0145), Natural Science Foundation of Hubei Province of China (Team Project, No. 2015CFA017) and the Special Fund for Environmental Protection in the Public Interest (2013467064). We thank Large-scale Instrument and Equipment Sharing Foundation of Wuhan University. We are grateful to Haiyan Huang (Chemical Biology of Nucleic Acids Lab, Wuhan University) and Siwei Zou (Natural Polymer and Polymer Physics Lab, Wuhan University) for the help of cytotoxicity assay.
Conflict of interest
There are no conflicts of interest to declare.