Abstract
The International Commission on Radiation Units and Measurements is considering revising the definitions of the operational dose quantities used for personal monitoring. This paper investigates the impacts of the proposed changes on the Public Health England two-element β/γ personal thermoluminescence dosemeter (TLD), in terms of its energy and angle dependences of responses for both skin and whole-body dose assessments. In general, the photon response of the skin element would be unaffected by the proposal, though technical issues may arise during calibration. For body photon doses, the current TLD design still produces acceptable response characteristics in some circumstances, but in general it will need to be redesigned to better match the requirements of the new operational quantity; to that end, a simple adaption is demonstrated that might provide a partial solution. For electron/beta exposures, matching the combined responses of both the body and skin elements to the dose quantities may be more challenging. The performance criteria against which dosemeters are judged may also need to be revised to reflect the proposed change.
Export citation and abstract BibTeX RIS
Introduction
At sites where photon or mixed photon-electron fields may be present, such as medical or industrial x-ray facilities, it is essential to monitor doses to personnel to ensure that safety regulations are being complied with and that risks to workers are kept as low as reasonably practical (ALARP). Typically, those doses are recorded by the routine wearing of personal dosemeters, either active devices that can provide real-time assessments with potential alarming capabilities, or passive dosemeters that may be worn for an agreed period before being sent back to accredited personal dosimetry services to be 'read-out'. Photon and electron personal dosemeters are designed to respond in terms of personal dose equivalent, Hp(d, θ, ε), which is defined by the International Commission on Radiation Units and Measurements (ICRU) as the dose at a depth of d mm in an ICRU 4-element tissue phantom from an exposure to photons or electrons of energy ε incident at angle θ (ICRU 1985). Values of d = 0.07, 3 and 10 mm are used to estimate Hp(d, θ, ε) relevant to the protection quantities equivalent dose to the skin, equivalent dose to the lens of the eye, and effective dose, respectively.
The personal dose equivalent per fluence, Hp(d, θ, ε)/Φ, and per air kerma, Hp(d, θ, ε)/Ka(ε), conversion coefficients were published by the ICRU and the International Commission on Radiological Protection (ICRP) in conjunction (ICRP 1996, ICRU 1998). For photons they were calculated using the kerma approximation, which is incorrect from a technical perspective due to fundamental differences between kerma and absorbed dose. However, when the current conversion coefficients were calculated, coupled electron-photon transport was not very well-developed within Monte Carlo software, and in any case the kerma conditions do not breakdown at 10 mm tissue depth for generated electrons until their energies exceed ∼2 MeV (ICRU 1984), so for most workplace fields the data were acceptable. Correct calculation of the quantity, using full secondary charged particle transport within Monte Carlo models in a vacuum, would prevent it from providing a proper estimate of the protection quantity effective dose, E, at higher energies because the ranges of the generated electrons would cause the maximum dose equivalent in tissue to be deposited at depths greater than 10 mm. Geometric differences between the ICRU surrogate phantoms and an anthropomorphic phantom are additional causes of discrepancy, with Hp(d, θ, ε)/Φ less than E(θ, ε)/Φ in some radiation fields; in such cases, Hp(d, θ, ε) is hence an inadequate quantification of the risk to individuals. In particular, Hp(10, θ, ε) can be a poor estimator of risk for exposures from angles >90°, where for example the personal dose equivalent per fluence, Hp(10, 180°, ε)/Φ, can be much less than the concurrent posterior-anterior (PA) effective dose per fluence, E(PA, ε)/Φ. On the other hand, for some fields Hp(10, θ, ε) provides an overly-cautious estimate of risk, such as for weakly penetrating photon exposures from anterior-posterior (AP).
In response to these limitations in the current definition of Hp(d, θ, ε), ICRU has recently considered its revision (Endo 2016, 2017), along also with redefinitions of the other operational quantities: ambient dose equivalent, H*(10, ε), and directional dose equivalent, H'(d, θ, ε). The proposed revision replaces: Hp(10, θ, ε) with a new quantity 'personal dose', defined in terms of effective dose; Hp(3, θ, ε) with a new quantity 'personal absorbed dose in the lens of the eye', defined in terms of absorbed doses to a stylised eye phantom; and Hp(0.07, θ, ε) with a new quantity 'personal absorbed dose in local skin', defined in terms of absorbed doses within a recommended region of skin. The symbols for these new definitions are Hp(θ, ε), Dp lens(θ, ε) and Dp skin(θ, ε), respectively. The primary motivation for these changes is to make the dose quantities better reflect the risks associated with a given exposure.
The suggested changes to the operational dose quantities are just a proposal at this stage, and ICRU have not yet issued their final report on the definitions. Nevertheless, it is inevitable that changing or abandoning Hp(d, θ, ε) could impact upon many systems of dosimetry, including the personal dosemeters that are designed to respond in terms of them. The present paper investigates how the proposed change would affect the Public Health England (PHE) two-element photon/electron personal thermoluminescence dosemeter (TLD) (Eakins et al 2007, Gilvin et al 2007), which assesses both Hp(10, θ, ε) and Hp(0.07, θ, ε) in mixed β/γ fields and is supplied to a wide variety of customers by PHE's approved Personal Dosimetry Service (PDS). The paper also considers how the current dosemeter might need to be redesigned to better match the response characteristics demanded by the new quantities.
The present work is intended neither to criticise nor support the ICRU proposals per se. Instead, its aim is simply to consider and discuss their potential impacts, and is hoped to complement recent publications by others (Otto 2018) and by the authors that similarly examined how the proposals would affect both the PHE neutron personal dosemeter (Tanner et al 2018) and a selection of neutron area survey instruments (Eakins et al 2018). Although the analyses here focus just on the PHE TLD, it may reasonably be assumed that many of the conclusions would also apply to other whole body β/γ personal dosemeters that are designed to respond similarly and in terms of the same operational dose quantities.
PHE whole-body TLD
The design and type-testing of the PHE whole-body TLD are described in detail elsewhere (Eakins et al 2007, Gilvin et al 2007). To summarise, however, it has two disc-like TLD-700H 7LiF: Mg, Cu, P detector elements held in a wrapped Harshaw card, which is encased within a holder with 2 mm thick polypropylene (PP) walls. The holder features an open hole in front of the Hp(0.07) element and an 18 mm diameter, 4.3 mm thick cylindrical polytetrafluoroethylene (PTFE) filter located inside the holder coaxially in front of the Hp(10) element. When calibrated on an ISO water-filled slab phantom, the dosemeter affords satisfactorily flat Hp(10) and Hp(0.07) relative response characteristics across the intended energy and angle ranges of applicability. Routine calibration of the TLD is performed in terms of 137Cs-equivalent air kerma.
For the purposes of this paper, and to avoid any potential confusion when different dose quantities are being discussed, the current 'Hp(0.07) element' of the TLD will be referred to as its 'uncovered element', and the current 'Hp(10) element' as its 'covered element'.
Conversion coefficients: definitions and comparisons
Proposed definitions
According to the proposal (Endo 2016, 2017) the personal dose Hp(θ, ε) at a given energy and angle will be set equal to the maximum of the corresponding effective doses at either +θ or −θ, i.e. MAX[E(θ, ε), E(−θ, ε)], where for plane parallel exposures θ is constrained to lie in a horizontal plane, i.e. perpendicular to the presumed head-to-feet 'axis' of the body. For rotational or isotropic exposures, Hp(θ, ε) ≡ E(θ, ε) by definition. The units of Hp(θ, ε) will therefore be sieverts, Sv. The fluence to personal dose conversion coefficient at a given energy and angle, Hp(θ, ε)/Φ, is hence obtainable from the maximum of the fluence to effective dose conversion coefficients at angles +θ and −θ, such as those derivable using the ICRP Reference Man and Woman voxel phantoms (ICRP 2009) and tabulated at selected angles in ICRP Publication 116 (ICRP 2010).
According to the proposed definition (Endo 2016, 2017) the personal absorbed dose in local skin, Dp skin(θ, ε), at a given energy and angle will be defined in terms of the absorbed dose deposited in a region located at a mean depth of ∼75 μm in a specified phantom, inside of which is a 2 mm thick outer layer of material representing skin of reference composition (ICRP 2002) and density 1.09 g cm−3 (ICRP 2010); the units of Dp skin(θ, ε) will therefore be grays, Gy. The choice of phantom, and shape and size of this region, will differ according to the intended location of wear of the dosemeter calibrated in terms of Dp skin(θ, ε), and hence according to what the phantom is intended to represent: the rod phantom for the finger; the pillar phantom for the extremities; and the slab phantom for the trunk of the body. For the PHE two-element whole-body TLD it is the trunk that is relevant, and Dp skin(θ, ε) is defined within a slab with outer dimensions 30 × 30 × 15 cm3 of ICRU 4-element tissue (density: 1.0 g cm−3), within a 2 mm layer of skin, in which the dose is averaged over the volume of a right circular cylinder with its axis perpendicular to the surface between the depths of 50 μm and 100 μm and a cross-sectional area of 10 mm2 below the centre of the front surface.
Personal dose: photons
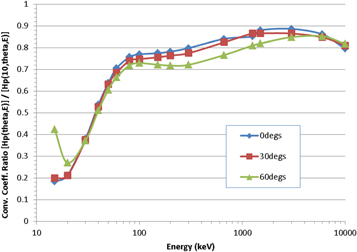
A comparison of Hp(10, θ, ε)/Φ and Hp(θ, ε)/Φ as a function of photon energy and angle of incidence is shown in figure 1, where the ratio rbody = Hp(θ, ε)/Hp(10, θ, ε) is plotted for a series of monoenergetic (15 keV to 10 MeV) and 137Cs (662 keV) and 60Co (mean energy: 1253 keV) sources, for plane-parallel exposures at 0° (i.e. anterior-posterior, AP), 30° and 60°. The data for Hp(10, θ, ε)/Φ are taken from ICRU Publication 57 (ICRU 1998).
Figure 1. Ratio, rbody, of the proposed new quantity Hp(θ, ε) to the current quantity Hp(10, θ, ε) for a range of photon energies and angles.
Download figure:
Standard image High-resolution imageIt is clear from the figure that Hp(10, θ, ε)/Φ always provides a reasonable estimate of effective dose within the energy and angle range plotted, though this is unlikely to be true for angles >90°. This aligns with the intended use of Hp(10, θ, ε) as a measurable surrogate for the protection quantity (ICRU 1985), though it is seen that at the lowest energy the effective dose is over-estimated by a factor of ∼5×. The observation is also as expected: in general, most radio-sensitive organs are located deeper than 10 mm in the body, and because the depth-dose profile is not uniform due to attenuation of the photons, differences between doses at 10 mm and doses to deep organs will be always be exhibited; moreover, because the gradient of the depth-dose profile varies inversely with photon energy, those differences will typically be greatest for low-energy exposures. Whilst it might be argued that it is better to over-estimate than under-estimate risk, it is clearly still an undesirable feature in the dose quantity, especially over this energy range that is typical of the workplace fields in which personal dosemeters are usually worn. On balance, therefore, the proposed quantity Hp(θ, ε) represents a more reliable assessment of potential risk to individuals than Hp(10, θ, ε), with the advantages particularly apparent in workplace fields featuring low-energy photons.
Personal absorbed dose in local skin: photons
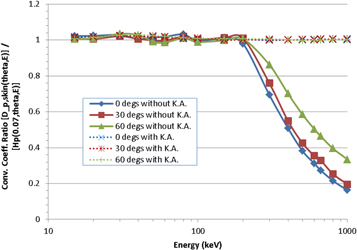
A comparison of Hp(0.07, θ, ε)/Φ and Dp skin(θ, ε)/Φ as a function of photon energy and angle of incidence is shown in figure 2, where the ratio rskin = Dp skin(θ, ε)/Hp(0.07, θ, ε) is plotted for a series of monoenergetic (15 keV to 1 MeV) and 137Cs (662 keV) sources, for plane-parallel exposures at 0° (i.e. anterior-posterior, AP), 30° and 60°. The data for Hp(0.07, θ, ε)/Φ were taken from ICRU Publication 57 (ICRU 1998), with 1 MeV the maximum energy provided therein.
Figure 2. Ratio, rskin, of the proposed new quantity Dp skin(θ, ε) to the current quantity Hp(0.07, θ, ε) for a range of photon energies and angles. The new quantity has been calculated both with (dashed lines) and without (solid lines) making the kerma approximation.
Download figure:
Standard image High-resolution imageIt is clear from the figure that Dp skin(θ, ε) and Hp(0.07, θ, ε) are essentially equivalent at low energies: they differ by no more than ∼3% across the energy range from 15 to 200 keV, for all three angles considered. At higher energies, however, larger divergences are observed between the current and proposed quantities, with Dp skin(θ, ε) becoming significantly lower than Hp(0.07, θ, ε). This deviation arises because the numerical values of the conversion coefficients given in ICRU 57 were calculated using the kerma approximation under the assumption of secondary charged particle equilibrium (CPE); they therefore begin to over-estimate absorbed dose to the skin at energies for which the range of the generated electrons is >0.07 mm, i.e. at energies above ∼70 keV at normal incidence, though the impact of this is only really observed above ∼200 keV. On the other hand, the data used for Dp skin(θ, ε) in figure 2 were not calculated using the kerma approximation, so discrepancies arise with Hp(0.07, θ, ε). The 50 to 100 μm depth range used for Dp skin(θ, ε), rather than 70 μm for Hp(0.07, θ, ε), also contributes to the form of figure 2. However, conversion coefficients for the new skin quantity have also been calculated under CPE conditions using the kerma approximation (Endo 2016, 2017), which might conveniently be labelled  (θ, ε)/Φ. If those data are instead compared against personal dose equivalent (figure 2), much better consistency is found: at 1 MeV, for example,
(θ, ε)/Φ. If those data are instead compared against personal dose equivalent (figure 2), much better consistency is found: at 1 MeV, for example,  (θ, ε) and Hp(0.07, θ, ε) can be shown to agree to better than 1% at each of 0°, 30° and 60°.
(θ, ε) and Hp(0.07, θ, ε) can be shown to agree to better than 1% at each of 0°, 30° and 60°.
Whether or not CPE is assumed, it may be concluded that adoption of the proposed quantity would impact the photon response of the uncovered element of dosemeters only negligibly from 15 to 200 keV (figure 2), which is the energy range of primary interest in assessing skin doses from soft x-rays and low-energy gammas. Moreover, if calibrated in terms of personal absorbed dose in local skin under the kerma conditions of secondary charged particle equilibrium, the response of the uncovered element is unlikely to be affected by the proposed changes across the entire 15 keV to 1 MeV energy range considered. Indeed, it might be argued that assuming CPE is preferable anyhow, being a more realistic representation of the likely conditions existing in most workplace photon fields (and calibrations); this strongly mitigates in favour of employing  (θ, ε)/Φ data rather than Dp skin(θ, ε)/Φ. But, if calibrated without build-up to ensure secondary charged particle equilibrium, the uncovered element could over-respond significantly at high energies. This could pose additional challenges in laboratory type-testing and routine calibration of dosemeters: in particular, routine calibration to 137Cs may require additional complications, such as precise specification and correction for source encapsulation and source-dosemeter separation, so calibration using a lower energy source or multiplication by a scaling factor may potentially prove preferable. It is anticipated that the International Organization for Standardization (ISO) and International Electrotechnical Commission (IEC) would have to resolve these issues before the new quantities were used in practice.
(θ, ε)/Φ data rather than Dp skin(θ, ε)/Φ. But, if calibrated without build-up to ensure secondary charged particle equilibrium, the uncovered element could over-respond significantly at high energies. This could pose additional challenges in laboratory type-testing and routine calibration of dosemeters: in particular, routine calibration to 137Cs may require additional complications, such as precise specification and correction for source encapsulation and source-dosemeter separation, so calibration using a lower energy source or multiplication by a scaling factor may potentially prove preferable. It is anticipated that the International Organization for Standardization (ISO) and International Electrotechnical Commission (IEC) would have to resolve these issues before the new quantities were used in practice.
Personal absorbed dose in local skin: electrons
Personal dose equivalent conversion coefficients for d = 0.07 mm are not provided in ICRU 57 for electrons. However, directional dose equivalent data, H'(0.07, θ, ε), are included at that depth, and these may be assumed to be numerically similar to Hp(0.07, θ, ε) for exposures from the front, i.e. θ = 0°. A comparison of H'(0.07, 0°, ε)/Φ and Dp skin(0°, ε)/Φ for electrons is therefore insightful and shown in figure 3, where the ratio rskin' = Dp skin(0°, ε)/H'(0.07, 0°, ε) is plotted for a series of monoenergetic electron sources from 70 keV to 2 MeV. It is clear from the figure that Dp skin(0°, ε) and Hp(0.07, 0°, ε) (≈ H'(0.07, 0°, ε)) are essentially equivalent at all but the lowest energies: they differ by no more than 5% across the energy range from 150 to 2000 keV. At low energies however, i.e. from ∼80 to ∼150 keV, large divergences are observed, which is likely caused by the impact of differing electron ranges on dose depositions with regards to the 70 μm depth at which Hp(0.07, 0°, ε) is defined and the 50 to 100 μm depth over which Dp skin(θ, ε) is defined. The volume of the scoring region for Dp skin(θ, ε) is also large compared to the ranges of those lowest energy electrons: effectively, the Dp skin(θ, ε) dose calculation may be 'diluting' their localised energy deposition over a large mass, relative to the H'(0.07, θ, ε) dose calculated at 70 μm. Analogous discrepancies between Dp skin(θ, ε) and Hp(0.07, θ, ε) would be expected at angles other than θ = 0°, albeit over slightly different energy ranges due to the energy-dependence of the electrons' Bragg peaks. Nevertheless, personnel monitoring in such low-energy, short-ranged beta radiation fields tends not to be a major application of two-element whole-body dosemeters, such as the PHE TLD. So, it may tentatively be inferred that the ICRU proposal would not impact the electron response of the TLD too deleteriously overall if its uncovered element were calibrated in terms of Dp skin(θ, ε). However, greater impact might be expected for dosemeters designed and worn to assess localised skin exposures from low energy beta-emitters, such as ring or fingerstall dosemeters.
Figure 3. Ratio, rskin', of the proposed new quantity Dp skin(0°, ε) to the current quantity H'(0.07, 0°, ε) for a range of electron energies.
Download figure:
Standard image High-resolution imageIn summary, the conclusion is that two-element body personal dosemeters such as the PHE TLD would in general likely still be adequate for measuring skin absorbed doses from beta particles. Consequently, the construction of the PHE TLD would not need to be altered from the current design if its uncovered element were required to respond in terms of Dp skin(θ, ε) rather than Hp(0.07, θ, ε), which might otherwise have been necessary to 'correct' for any changes to its response characteristics.
Personal dose: electrons
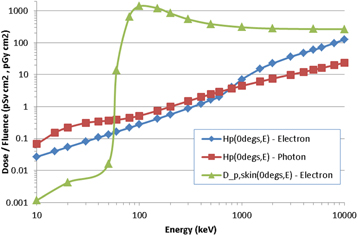
The covered element of the current PHE dosemeter is intended to be effectively insensitive to beta particles. This is because most beta sources encountered in the workplace are relatively low in energy, and electrons with energies below ∼2 MeV are unable to penetrate to a depth of 10 mm in tissue (ICRU 1984) so do not contribute directly to Hp(10). Accordingly, range-energy tables were employed during the design stage of the PHE TLD to ensure that its PTFE + PP filter effectively stops electrons below this energy (Eakins et al 2007). However, the proposed quantity personal dose is not zero for electrons with energies below 2 MeV, because low-energy electrons will always deposit skin doses and will hence always contribute to effective dose (and therefore Hp(θ, ε)), albeit reduced by a tissue weighting factor of 0.01. As a consequence, rather than being null, the Hp(θ, ε)/Φ conversion coefficients for low-energy electrons instead very approximately match those for low-energy photons, as shown in figure 4 for 0°, which is the limiting exposure geometry for Hp(θ, ε)/Φ for electrons (Endo 2016, 2017). Any dosemeter that is intended to monitor Hp(θ, ε) for β/γ exposures ought therefore to be able to respond both to low-energy electrons and photons. The current design of PHE TLD would clearly fail to meet this criterion for common beta sources, such as 147Pm, 85Kr and 90Sr/90Y, for which it would respond negligibly as required for Hp(10).
Figure 4. Hp(0°, ε)/Φ conversion coefficients for electrons and photons, and Dp skin(θ, ε)/Φ for electrons.
Download figure:
Standard image High-resolution imageDesigning a single element Hp(θ, ε) dosemeter that responded equally well to both β and γ exposures across a wide energy range would be difficult: essentially, measurement of electrons would demand very little filtration, but a thick filter (e.g. ∼10 mm tissue-equivalence) is required to simulate the attenuation of more penetrating photons that deposit doses to organs located deep within the body. However, a two-element dosemeter need not necessarily exhibit this limitation if considered in combination: skin doses, which are the main contributor to effective dose from beta exposures, could potentially be monitored by the uncovered element. A non-negligible filter could therefore be retained in front of the covered element, because information on the contribution to Hp(θ, ε) from low-energy betas could conceivably be accounted for implicitly by the TLD's other element. In such a case, careful calibration and subtraction could be required to avoid-double counting of the photon component to dose that could concurrently be deposited in that second element.
Also shown in figure 4 are the fluence to personal absorbed dose in local skin conversion coefficients for electrons; of course Dp skin(θ, ε) and Hp(θ, ε) have different units, Gy and Sv, respectively, and are intended to quantify different risks, tissue reactions and stochastic risks, respectively, but for photons and electrons they may be assumed numerically equivalent. The values for Dp skin(θ, ε)/Φ and Hp(θ, ε)/Φ were also determined differently (Endo 2016, 2017), with electron doses in the latter calculated by dividing the energy deposited in the ∼2 mm thick voxels that represent the skin by their combined mass. This explains why the ratio Dp skin(θ, ε)/Φ: Hp(θ, ε)/Φ varies by amounts different from simply 100:1, which might naively be assumed from considering just the 0.01× tissue weighting factor. Electrons with energies below ∼60 keV are unable to reach the 50 to 100 μm deep region used to determine Dp skin(θ, ε), but are still able to deposit doses in the voxels; indeed, electrons will always deposit more energy in the voxel than in the 50 to 100 μm deep region, because of the energy deposited in the first 50 μm. Moreover, the position of the electron's Bragg peak, and specifically whether it lies at a depth between 50 and 100 μm or well beyond that range, as well as the different masses of the two regions over which the two doses are averaged, both lead to the Dp skin(θ, ε)/Φ: Hp(θ, ε)/Φ ratio varying either above or below 100: 1.
Figure 4 shows that Hp(θ, ε)/Φ for electrons is never the largest quantity in this energy range, compared to Hp(θ, ε)/Φ for photons and Dp skin(θ, ε)/Φ for electrons. However, the skin dose limit is 500 mSv and the whole body dose limit only 20 mSv, so there is a factor of 25× in the way in which the quantities are applied. In practice, this means that the skin dose will be limiting for electrons when it is more than a factor of 25× larger than personal dose for electrons, which is the case for electrons with energies between 60 keV and 1.5 MeV (figure 4). Although Dp skin(θ, ε)/Φ is less than Hp(θ, ε)/Φ for electrons with energies below ∼60 keV, these have a range in air of <6 cm (ICRU 1984), so are essentially irrelevant for whole body dosimetry, and in any case would be unlikely to be detected by the body dosemeter unless it happened to be located ideally. Moreover, the actual Hp(θ, ε) doses deposited by electrons are small at those energies, being ∼2 orders of magnitude lower than at 1 MeV, for instance (figure 4). At energies above ∼2 MeV, Hp(θ, ε)/Φ for electrons is greater than that for photons, but these energies are not directly relevant for common beta sources.
The difference between Dp skin(θ, ε) and Hp(θ, ε) raises an important question: what should the 'skin element' (i.e. the uncovered element) of the TLD measure if the proposed quantities were accepted? The region from 50 to 100 μm in skin was adopted because it approximates the location of the basal cells of the epidermis, which are the skin tissue at radiogenic risk (ICRP 2010). It might therefore be argued that averaging doses over an entire ∼2 mm thick voxel is not an optimal means of assessing stochastic risks from low-energy electrons. So, whilst Hp(θ, ε) might be considered preferable to Hp(d, θ, ε) for neutral particles and for very high-energy, very highly penetrating charged particles that can deposit doses deep within the body (such as might be encountered in cosmic ray or accelerator dosimetry for instance), it may prove to be a less appropriate quantity for assessing stochastic risks from low-energy electrons, such as beta particles. Indeed from this argument, Hp(θ, ε) might also be viewed as less appropriate for electrons than Dp skin(θ, ε), even though personal absorbed dose in local skin (in Gy) is intended to assess risks from tissue reactions. On the other hand, it might naturally be assumed that the 'body' element of a personal dosemeter would be expected to respond in terms of Hp(θ, ε) if the proposed quantities were adopted; if electrons are contributing to that quantity, a whole body β/γ dosemeter ought therefore to respond adequately to beta sources in terms of personal dose, rather than just dose to local skin. But, that would be difficult to achieve in practice using a single covered element, without concurrently degrading its photon Hp(θ, ε) response.
It might be argued that whole-body exposures to plane-parallel beta sources are unlikely in most practical workplace fields. Low-energy electrons straggle considerably, and also have very short ranges: a 100 keV electron has a range of only ∼0.14 m in air, for instance, though this increases to ∼4 m and ∼9 m for 1 MeV and 2 MeV electrons respectively (ICRU 1984). Further, the beta-emitting radionuclide itself is likely to be spatially confined, leading to a highly divergent field unless located a large distance from the individual. The lack of an Hp(θ, ε) response for betas by the covered element might therefore be a somewhat artificial limitation, which may only be a problem in principle, but would not lead to significant under-estimates of overall risks in realistic workplace fields. In that regard, the precise position where the dosemeter is worn may be a more significant contribution to uncertainty than the lack of a β response in the covered element, with the inverse-square dose variations that are caused by the divergence of a beta point-source perhaps more likely to lead to under- or over-estimations of overall skin doses. Likewise, using a whole body dosemeter to monitor Dp skin(θ, ε), which is a quantity that is intended just to quantify localised tissue reactions in skin, could greatly misassess risk; since those exposures are mainly encountered just in extremity dosimetry, it could again be argued that the two-element PHE TLD ought not be used to estimate the personal absorbed dose in local skin.
The above supports the suggestion that if the proposed dose quantities were accepted, such that Hp(10, θ, ε) and Hp(0.07, θ, ε) became superseded, the use of a two-element whole body dosemeter could become redundant unless the uncovered element were instead somehow repurposed for assessments of the electron component to Hp(θ, ε). However, this suggestion is complicated by the observations discussed earlier regarding the definition of Hp(θ, ε), specifically that it is calculated by averaging doses over an entire voxel rather than just at the depths of radiosensitive regions within the skin. Moreover, the ∼2 order-of-magnitude variation in Hp(θ, ε) across the energy range relevant to beta dosimetry (figure 4) may make its assessment by a single, unfiltered LiF: Mg, Cu, P element somewhat challenging.
Overall, it is unclear at present what the preferred use or intended function of the uncovered element of the two-element TLD should be optimally if Hp(θ, ε) and Dp skin(θ, ε) were both adopted. Further clarification from ICRU on this issue might therefore be required, along with guidance on the recommended approach for electron and beta dosimetry in the context of the proposed local and whole body dose quantities.
Method: Monte Carlo modelling
The overall aim of the current work is to determine the energy- and angle-dependent response characteristics of the PHE TLD using Hp(θ, ε) and Dp skin(θ, ε) data in place of Hp(d, θ, ε) data, to understand whether it will still perform acceptably or else require modification. Calculations were performed using the Monte Carlo code MCNP6 (Pelowitz 2013).
For photons, the personal dose equivalent response characteristics of the TLD have been calculated previously (Eakins et al 2007) as a function of energy from ∼10 keV to several MeV, using appropriate Hp(10) per air kerma data and air kerma per fluence data (ISO 1999, Ankerhold 2000). Those results: related to simulated exposures to the ISO Narrow Series fields (ISO 1996) and selected radionuclide sources; were derived using the Monte Carlo code MCNP4c2 (Briesmeister 2000); and were benchmarked against laboratory type-test measurements in those fields (Gilvin et al 2007). For the present work, the dose response characteristics have been recalculated using the updated version MCNP6, for exposures to 137Cs (662 keV), 60Co (mean energy: 1253 keV) and a series of monoenergetic (15 to 300 keV) sources. Apart from providing more accurate results due to the use of updated photon cross-section libraries (Eakins 2009), recalculation also permits the adoption of a common energy grid for comparing the responses obtained by using the current and proposed operational quantity conversion coefficients. In all calculations, the TLD was positioned on the front face of a 30 × 30 × 15 cm3 ISO water-filled slab phantom, which mimics the recommended laboratory calibration conditions and is intended to represent the positioning of the TLD on the trunk of the body during routine wear.
For the angle dependence, effort was focussed on plane-parallel exposures at 0° (i.e. anterior-posterior, AP), 30° and 60°, and rotationally (ROT) and isotropically (ISO) symmetric fields; in common with the ICRU approach (Endo 2016, 2017), a true rotational field was modelled rather than any approximation (Eakins and Kouroukla 2015). The maximum of 60° was chosen because of its relevance to the criteria against which TLDs are currently judged (IEC 2012), whilst ROT and ISO are assumed to be the most relevant fields to many realistic workplace exposures. In all cases, an energy-dependent relative thermoluminescence efficiency function, η'(ε), was applied to the calculated absorbed doses deposited in the LiF: Mg, Cu, P element, with interpolation of the η'(ε) dataset performed at intermediate energies when required, in order to relate them to the responses expected to be exhibited during routine read-out of the TLD (Eakins et al 2007). Of course, when comparing the TLD's Hp(θ, ε) response to its Hp(10, θ, ε) response at the same energy ε, these applications of η'(ε) cancel out.
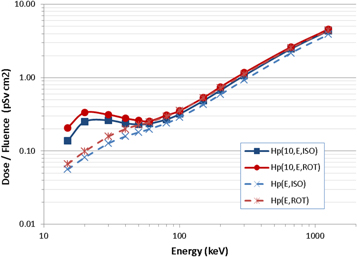
All of the Dp skin(θ, ε) and Hp(θ, ε)/Φ conversion coefficients were taken directly from the ICRU proposal, which was freely available as a download for consultation on the ICRP website (Endo 2016, 2017). The Hp(10, θ, ε)/Φ data for θ = 0°, 30° or 60° were taken from ICRU Publication 57 (ICRU 1998), but conversion coefficients for ROT or ISO exposures are not provided in that document. For those fields, Hp(10, ISO, ε)/Φ and Hp(10, ROT, ε)/Φ data were therefore calculated by Monte Carlo modelling, using MCNP to simulate exposures of an ICRU four-element slab phantom in vacuo, with photon kerma tallied in a 0.1 mm thick, 5 mm radius cylindrical region located at a mean depth of 10 mm from the centre of the phantom's front face; fluence normalisation was achieved by repeating the simulations with all materials changed to air or vacuum, and tallying fluence through the cylinder. Benchmarking of the model was achieved by performing selected plane-parallel exposures from forward angles and comparing the results with data provided in ICRU 57; general consistency with the ICRU data was verified, though exact agreement cannot be expected anyhow because of differences in MCNP and the cross-section data, for example. The Hp(10, ROT, ε)/Φ and Hp(10, ISO, ε)/Φ results that were obtained are presented in figure 5, compared against the analogous data for personal dose, Hp(ROT, ε) and Hp(ISO, ε), which are numerically equal to the data for effective dose, i.e. E(ROT, ε)/Φ and E(ISO, ε)/Φ. As can be seen, and similarly to the patterns exhibited for plane-parallel exposures (figure 1) and for the same reasons, Hp(10, ROT, ε) > Hp(ROT, ε) and Hp(10, ISO, ε) > Hp(ISO, ε) across the entire energy range considered, with the differences greatest at low energies as anticipated.
Figure 5. Hp(10, θ, ε)/Φ and Hp(θ, ε)/Φ conversion coefficients for ISO and ROT photon exposures.
Download figure:
Standard image High-resolution imagePhoton results: Hp(10, θ, ε) versus Hp(θ, ε)
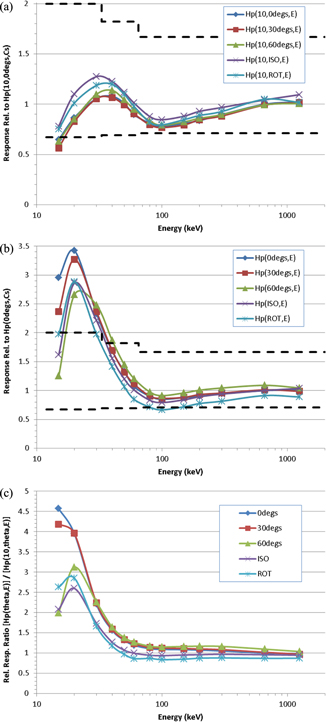
The Hp(10, θ, ε) responses of the TLD are shown in figure 6(a) relative to its Hp(10, 0°, Cs) response, with this normalisation chosen to match the dosemeters' routine calibration. The Hp(θ, ε) responses are shown in figure 6(b) relative to the Hp(0°, Cs) response, demonstrating the impact on the TLD of the proposed change to the operational dose quantity. These two datasets are further compared in figure 6(c), which plots the ratios [Hp(θ, ε)/Hp(0°, Cs)] ÷ [Hp(10, θ, ε)/Hp(10, 0°, Cs)] as a function of energy and orientation. The statistical uncertainties on all Monte Carlo data were no more than a few %, so have been omitted from these figures (and likewise elsewhere in this paper) for clarity.
Figure 6. (a). Photon Hp(10, θ, ε) response of the TLD relative to Hp(10, 0°, Cs). The limits recommended by the IEC for personal dose equivalent are also shown (dashed lines). (b). Photon Hp(θ, ε) response of the TLD relative to Hp(0°, Cs). The limits recommended by the IEC for personal dose equivalent are also shown (dashed lines). (c). The ratio [Hp(θ, ε)/Hp(0°, Cs)] ÷ [Hp(10, θ, ε)/Hp(10, 0°, Cs)] for photons for the PHE TLD.
Download figure:
Standard image High-resolution imageA peak in the relative response curve at a few 10 s of keV, and a trough at ∼100 keV, are exhibited in both figures 6(a) and (b), and are ultimately traceable to the tissue-inequivalence of the LiF: Mg, Cu, P material. The deviation from a flat energy-dependence of response is considerably greater for Hp(θ, ε) than for Hp(10, θ, ε), however, and overall it is clear from figure 6 that changing the dose quantity would significantly affect the relative response characteristics of the dosemeter, with the greatest impact occurring at low energies and for exposures from the front. This is anticipated from figures 1 and 5 and their interpretation, and from the observation that the material that covers the body element of the TLD (=4.3 mm of 2.2 g cm−3 PTFE + 2 mm of 0.9 g cm−3 PP), which was chosen because its effective Z and combined mass-thickness of ∼1.1 g cm−2 make it roughly equivalent to 10 mm of ICRU four-element tissue in terms of attenuation of the primary photon beam, is inadequate to properly mimic the attenuation of the beam at the depths of the organs that are key to effective dose, in terms of which the new quantity is defined.
Conversely in ROT fields, and to a lesser extent ISO fields, the relative response of the dosemeter would be lower for Hp(θ, ε) than for Hp(10, θ, ε) when normalised to the routine calibration response to 137Cs at 0°, though the differences also exhibit a strong energy dependence. This is because half the fluence in those fields is from the rear, and this component is typically attenuated more when it passes through the slab phantom en route to the TLD than in the analogous case in which it deposits doses in organs of the body; essentially, the ≥15 cm thickness of water and polymethyl methacrylate (PMMA) that comprises the calibration phantom acts as a filter that is too effective relative to the amount of tissue separating most organs from the source. Of course, this observation for ROT is a consequence of wearing the TLD at a single location on the front of the body, rather than a limitation in the proposed dose quantity itself. Nevertheless, it does highlight a potentially intractable difficulty in designing dosemeters with response characteristics that match the desired dose quantity.
Response criteria
Photons
The performances of photon/electron personal dosemeters are typically judged against criteria stipulated in international standards, such as those recommended by the IEC; the current standard (IEC 2012) supersedes the previous version (IEC 2006), which the PHE TLD was originally designed to meet, though their differences in requirements for Hp(10, θ, ε) response characteristics are relatively minor. Amongst other criteria, the IEC standard recommends that the TLD be type-tested using the ISO Narrow Series fields (ISO 1996), as well as 137Cs and 60Co radionuclide sources, and that in the angle range −60° ≤ θ ≤ +60° its relative responses should lie: between 0.67 and 2.00 for 12 ≤ εm < 33 keV; between 0.69 and 1.82 for 33 ≤ εm < 65 keV; and between 0.71 and 1.67 for εm ≥ 65 keV, where εm denotes the mean energy of the photon field. These recommended limits have been superimposed onto figures 6(a) and (b) for illustration, though it is reiterated that they do not apply for the ROT and ISO exposures. Clearly, the recommendations are met for the Hp(10, θ, ε) relative response of the PHE TLD at all energies above ∼20 keV, with the responses at 15 keV only just overstepping the criterion, notwithstanding that the calculated response data are for monoenergetic sources rather than a distribution. However, in general the criteria are not met for the Hp(θ, ε) relative response at energies below ∼50 keV.
Several comments may be made regarding the above observation for the Hp(θ, ε) relative response. Firstly, the energy range considered 'mandatory' in the standard is only from 80 keV to 1.25 MeV, and the Hp(θ, ε) relative response is not in violation of the recommendations within these limits. Nevertheless, the rated energy range of the PHE TLD is currently broader than this restricted range, so not complying over that whole span is undesirable: when used in workplaces with significant components of the field below 80 keV, the dosemeter would be expected to respond across the full intended energy range.
Secondly, the overall asymmetry in the over- versus under-response across the entire energy range may be improved a little by recalibrating using a source other than 137Cs, or by applying a constant scaling factor, to 'shift' the whole response downwards. For example, calibrating instead to the Hp(0°, 60 keV) response, or multiplying the Hp(0°, Cs) result by ∼1.15× prior to normalizing the other responses to it, would both serve to reduce the over-response at low energies whilst maintaining compliance with the minimum relative response requirements at all other energies. However, these two options are just compromises: whilst recalibration may improve relative responses overall, and in turn lower the energy range of compliance from ∼50 keV to ∼40 keV, it would be insufficient to address the large over-response peaked at 20 keV, so cannot alone solve all of the limitations that would be introduced for the current design of TLD upon adopting the new dose quantity. In addition, use of Hp(0°, 60 keV) as a routine calibration source, or even the N80 field that has a mean energy of ∼60 keV (ISO 1996), would be less convenient than a readily available radionuclide such as 137Cs.
Thirdly, however, and conversely to all of the above, it is noted that because the IEC criteria are given in terms of the Hp(10, θ, ε) response, it may be argued that they would not be an appropriate test for dosemeters calibrated to Hp(θ, ε). This leads naturally to the suggestion that changing the dose quantity from Hp(10, θ, ε) to Hp(θ, ε) would inevitably require corresponding amendments to those standards against which personal dosemeters are judged. Of course, guidance on assessing the under-responses of the TLD to ROT and ISO fields, which is common in both Hp(10, θ, ε) and Hp(θ, ε) responses but worse for the latter, would also ideally be addressed in any revised standard; the current practice of calibration on a slab phantom presents geometric difficulties for exposures for angles >85° in terms of personal dose equivalent, but these are not problems with personal dose, so the IEC might choose to add performance requirements for specific reverse angles if personal dose were adopted.
Electrons
It is not possible to predict what the performance limits might be for personal dosemeters if the new operational quantities were introduced, but the current requirements for performance of whole body dosemeters in terms of Hp(0.07) only specify a range from 0.71–1.67 for exposure to 90Sr/90Y at normal incidence (IEC 2012). Extremity dosemeters have more onerous performance requirements for energy and angle dependence of response, but the relatively large distances between the source and the torso make lower energy betas less relevant for whole body dosimetry.
Potential improvements for the PHE TLD
Dosimetrically, the PHE TLD features attenuating material in front of its LiF: Mg, Cu, P body element that comprises a 4.3 mm thick PTFE filter and 2 mm of PP. Practically, the PTFE filter is an 18 mm diameter cylinder (i.e. large enough to still provide adequate coverage at ±60° angles) that is fitted tightly into a cylindrical recess in the TLD's holder, which is constructed from 2 mm thick PP; such a fabrication enables relatively simple and inexpensive manufacture. This filter combination was originally investigated and optimised during the design stage of the dosemeter using MCNP4c2 to give it the best possible relative Hp(10, θ, ε) response (Eakins et al 2007), without compromising the Hp(0.07, θ, ε) response by causing any 'shadowing' of that second element. If the proposed dose quantity were adopted, an obvious question is whether modifying its filter configuration might allow for better matching of the energy- and angle-dependent response characteristics required for Hp(θ, ε). A full such re-optimisation campaign is beyond the scope of the current paper and, in any case, would be premature before publication of the final recommendations by ICRU and interpretation by IEC. Nevertheless, it is insightful to consider the possible directions in which such redesign work might progress and what might potentially be achieved.
Photon dosimetry
A simple redesign procedure was performed for the PHE TLD using the Monte Carlo code MCNP6, with the dosemeter again located on an ISO water-filled slab phantom. The aim was to converge upon an adapted filter that could be used within the current PP holder, which was presumed to be the most cost-effective solution. Moreover, by choosing this constraint that any redesign should be externally identical to the current TLD, it can be assumed that doses to the uncovered element of the dosemeter would be relatively unaffected by any modification; if a much thicker overall design were instead proposed, for instance, some 'shadowing' could potentially arise. Thus, hybrid filters were considered as the simplest first step, consisting of x mm of metal plus (4.3-x) mm of PTFE: it was speculated that incorporating a material with a greater effective-Z and/or density than PTFE would supress the over-response in the low-energy (i.e. photoelectric) range (figure 6(b)).
The general methodology for the redesign process was iterative, varying the material, thickness and relative locations of the filter components, and calculating and comparing the Hp(θ, ε) relative response characteristics for θ = 0°, 30°, 60°, ROT and ISO exposures to a series of monoenergetic (15 to 300 keV) and 137Cs and 60Co sources; for a given configuration, normalisation to its calculated Hp(0°, Cs) response was applied. Aluminium, copper and tin filters were considered, with thicknesses from 0.2 to 1.5 mm, as these provided a range of different atomic numbers (Z = 13, 29 and 50, respectively), are higher in density (∼2.68, 8.96 and 7.31 g cm−3 respectively) than PTFE, and are each readily and cheaply available.
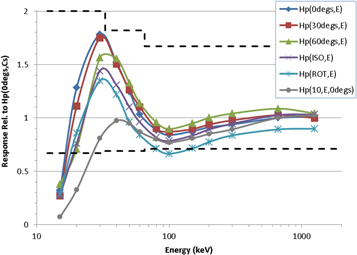
The full details and results from all of these trials are not included here for brevity. Out of the variants considered, the optimum design featured a 1.5 mm thick, 18 mm diameter aluminium cylinder located between the 2 mm thick PP wall of the holder and the PTFE cylinder, the thickness of which had been reduced accordingly to 2.8 mm. The relative responses of this redesigned dosemeter are shown in figure 7. For the 137Cs exposure, the absorbed dose to the LiF: Mg, Cu, P per applied air kerma in the redesigned model was found to be 0.99 Gy Gy−1, which is the same as the value for the current design of TLD, indicating that the modification would not cause any significant loss of sensitivity. By consulting range-energy tables (ICRU 1984), it was also shown that the currently required insensitivity of the covered element to most β-particle sources would also not be compromised by the modification. For completeness, the Hp(10, 0°, ε)/Hp(10, 0°, Cs) performance of this new design is also shown in figure 7, which displays a significant under-response at low-energies as expected.
Figure 7. Photon Hp(θ, ε) response of the redesigned TLD relative to its Hp(0°, Cs) response. Also shown is the Hp(10, 0°, ε)/Hp(10, 0°, Cs) relative response of that redesign, and the limits recommended by the IEC for personal dose equivalent (dashed lines).
Download figure:
Standard image High-resolution imageSuperimposed on figure 7 are the performance limits recommended by IEC for personal dosemeters (IEC 2012). Notwithstanding that these are defined in terms of personal dose equivalent rather than personal dose, so would likely be revised if the proposed dose quantity were accepted, it is encouraging to note that the redesigned dosemeter complies with the current standard across the entire energy range apart from at 15 keV, where it still under-responds by a large degree. Although technically these limits do not apply for ROT or ISO exposures, it is also interesting to note that the responses to these fields would also comply with the standard apart from at 15 keV and for ROT at 100 keV. Moreover, if a constant scaling factor of 1.1× were applied during calibration, this latter ROT response would then meet the 0.71 minimum without causing the other responses to non-comply: the worst-case relative over-responses, i.e. Hp(0°, 30 keV) and Hp(30°, 30 keV), would still be less than the recommended limit of 2.00.
Electron dosimetry
The uncovered element of the TLD was unchanged in the above redesign process, so should generally respond well to Dp skin(θ, ε) (and Hp(0.07, θ, ε)) for both photons and betas. As discussed earlier, however, designing a dosemeter with a single covered element that responds equally well to Hp(θ, ε) for both electron and photon exposures across a wide energy range would be problematic; indeed, designing a single element dosemeter with an adequate Hp(θ, ε) response for just electrons would be challenging. To illustrate the difficulty, the Hp(0°, ε) responses of the redesigned TLD to monoenergetic electrons from 1 to 20 MeV have been calculated using MCNP6 for both the covered and uncovered elements. The results are shown in figure 8 given relative to their respective Hp(0°, Cs) responses, though in fact those calibrations only differ by <3%. It is seen that whilst the covered element greatly under-responds below ∼2 MeV, it greatly over-responds between ∼3 MeV and ∼10 MeV. Conversely, the uncovered element always over-responds. In both cases, only above 15 MeV is the relative response within ∼50% of unity. Thus, it is clear from figure 8 that the covered element in the redesigned TLD, with its aluminium insert, does not resolve the issue for electrons like it did for photons. In fact, neither element of the redesigned dosemeter could be used to directly assess Hp(θ, ε) for electrons for the entire range of energies for which conversion coefficient data are provided in the ICRU proposal.
Figure 8. Electron Hp(0°, ε) responses of both the covered and uncovered elements of the redesigned TLD relative to their respective Hp(0°, Cs) responses. Filled symbols denote monoenergetic exposures, whilst empty symbols denote beta exposures plotted at their mean energies: ∼0.05 MeV for 147Pm, ∼0.2 MeV for 85Kr and ∼0.3 MeV for 90Sr/90Y.
Download figure:
Standard image High-resolution imageIt might be remarked that plane-parallel exposures of individuals to a wide range of monoenergetic electron sources are an unlikely scenario for practical radiation protection in the workplace. Instead, responses to β or mixed β/γ fields are perhaps more relevant, in which electrons typically exhibit a distribution of energies with a relatively low mean. With this refocus, it might be speculated that the proposed redesign of two-element TLD could still potentially be capable of assessing both Dp skin(θ, ε) and Hp(θ, ε) in mixed β/γ fields if some changes were made to its dose algorithm. No electrons with energies below ∼2 MeV would be detected by the covered element of the redesigned dosemeter; but, those electrons could be measured effectively by the uncovered element of the dosemeter. The solution might therefore be to reclassify the two-element dosemeter as capable of assessing 'skin' doses and the beta component of Hp(θ, ε) with one of its elements, and the γ component of Hp(θ, ε) using the second element. Indeed, in workplace fields known to contain beta sources, potentially two calibration factors could be applied to the readout from the uncovered element: one to convert the measured TL to an assessment of Dp skin(θ, ε), and one to convert it to an assessment of Hp(θ, ε) for betas. Of course, these conversion factors would have different magnitudes and units, Gy−1 and Sv−1 respectively, and careful calibration and subtraction may also be required to avoid double-counting of the photon component to Hp(θ, ε) that would concurrently be deposited in the uncovered element. Careful sensitivity analyses would also need to be performed on the algorithm to avoid incorrect dose assessments caused by any improper weighting of the different components across the energy range. Furthermore, if this option were pursued, dosemeter performance requirements, such as those recommended by the IEC, might need to be amended to clarify the reclassification.
To explore the above considerations, the responses of the elements of the dosemeter have also been calculated using MCNP for exposures to 147Pm, 85Kr and 90Sr/90Y beta sources at 0°, notwithstanding the earlier comments that plane-parallel beta fields are somewhat artificial, especially at low energies, and that exposures in a vacuum neglect the inevitable attenuation of the field by air in a real workplace. The energy-distribution spectra of the radionuclides were obtained from the RADAR resource (RADAR 2018). Conversion coefficient data were derived by convolving the beta spectra with the available monoenergetic Dp skin(0°, ε)/Φ and Hp(0°, ε)/Φ data (Endo 2016, 2017), and are provided in table 1 along with the TLD response results for the uncovered element. For completeness, the relative Hp(0°, ε) response results of both elements are also plotted in figure 8 at the mean energies of the fields (∼0.05 MeV for 147Pm, ∼0.2 MeV for 85Kr, and ∼0.3 MeV for 90Sr/90Y), noting that this is for convenience but is also somewhat misleading: for 90Sr/90Y, for instance, most of the dose is deposited by its high energy components, which extend significantly beyond 0.3 MeV up to ∼2.2 MeV.
Table 1. Dp skin(0°, ε)/Φ and Hp(0°, ε)/Φ conversion coefficients, and uncovered TLD element responses, for 147Pm, 49Kr and 90Sr/90Y beta sources.
| Beta Source | Dp skin(0°, ε)/Φ (Gy cm2) | R[TLD/Dp skin(0°, ε)] | Hp(0°, ε)/Φ (Sv cm2) | R[TLD/Hp(0°, ε)] |
|---|---|---|---|---|
| Pm-147 | 5.15 × 10−10 | 0.016 | 1.97 × 10−13 | 41.6 |
| Kr-85 | 5.87 × 10−10 | 0.56 | 7.09 × 10−13 | 467 |
| Sr-90/Y-90 | 5.55 × 10−10 | 0.57 | 2.20 × 10−12 | 144 |
It is seen in table 1 that for Dp skin(0°, ε) the uncovered element of the TLD responds poorly for 147Pm, likely because the very low energy electrons from that source are absorbed by the thin wrappings surrounding the sensitive LiF: Mg, Cu, P element. However, the mean energy of electrons in the 147Pm distribution is ∼50 keV and their maximum is ∼200 keV, which will have ranges in air of ∼4 cm and ∼40 cm respectively (ICRU 1984), so 147Pm is unlikely to be particularly relevant to realistic whole body exposures in the workplace.
For 85Kr and 90Sr/90Y the uncovered element of the TLD also under-responds to Dp skin(0°, ε), but by less than 50% in both cases; moreover, if recalibrated in terms of 90Sr/90Y the relative response for 85Kr would be ∼0.98. The uncovered element of the TLD over-responds greatly to Hp(0°, ε) for 147Pm, 85Kr and 90Sr/90Y, but again if recalibrated in terms of 90Sr/90Y, the relative responses for 147Pm and 85Kr would 'only' under- and over-respond, respectively, by a factor ∼3×. Though ultimately still unsatisfactory, and well outside of the limits specified in the current performance requirements for Hp(0.07, θ, ε) (IEC 2012), these exceptions might be the best that could be achieved, so might potentially have to be considered acceptable for pragmatism with the caveat that whole body beta exposures in the workplace are perhaps unlikely to be a very large component to an individual's overall dose. Again, guidance and considerations on this would be required from IEC before the TLD could be employed in this way.
Of course, changing the calibration source for the uncovered element from the current 137Cs to 90Sr/90Y would have consequences for its concurrent measurement of photons. The Dp skin(0°, 90Sr/90Y) response of the uncovered element of the TLD can be shown to be ∼0.19× lower than its Dp skin(0°, 137Cs) response; conversely, its Hp(0°, 90Sr/90Y) response is ∼140× higher than its Hp(0°, 137Cs) response. Whichever dose quantity the uncovered element were calibrated in terms of, its responses to betas and X-rays would therefore be very different, drastically so for personal dose and easily explainable from a consideration of the penetrating nature of 662 keV photons compared to the much shorter ranged electrons.
Overall, then, the very different measurement and dosimetric requirements for electrons and photons, and their different calibration requirements, may mean that the uncovered element of the redesigned dosemeter could only be used in mixed photon/electron environments when the field was sufficiently well-characterised such that the anticipated dose contributions from the two components may be estimated in advance and the presence of higher-energy electrons can be ruled out; this might permit the use of appropriate weighting factors to deconvolve their respective contributions from the single TL signal that is read-out. This might lead to a scenario in which workplace field-specific correction factors need to be applied. Though clearly a disadvantage relative to the 'universal' calibration presently adopted for routine-issue TLDs, such a practice can be relatively common in neutron workplace fields (Hager et al 2017) so would not necessarily represent a complete departure from currently accepted practice in personal dosimetry.
Summary
There are energies and angles for which Hp(d, θ, ε) either significantly over-estimates or under-estimates the risk to individuals in photon, beta, or mixed photon/electron fields, providing motivation for the change to the operational dose quantity proposed by ICRU. It is inevitable, however, that designing and calibrating dosemeters in terms of these new quantities could lead to response characteristics that are significantly different from those obtained when designed and calibrated in terms of Hp(d, θ, ε). Although the analyses of this presented in the current work have focussed just on the PHE TLD, it may be assumed that many of the conclusions would also apply similarly to other whole body β/γ personal dosemeters, because they have been designed to respond to the same dose quantities according to the same performance criteria (IEC 2012).
The proposed quantity personal absorbed dose in local skin, Dp skin(θ, ε), is sufficiently similar to Hp(0.07, θ, ε) for photons at energies below ∼200 keV (figure 2), or ≲1 MeV if the kerma approximation is used, to conclude that the response of the uncovered element of the PHE TLD would be relatively unaffected by the ICRU proposal, though careful consideration may be required during calibration regarding whether secondary charged particle equilibrium should be assumed. On the other hand, the proposed quantity personal dose, Hp(θ, ε), differs significantly (figure 1) from Hp(10, θ, ε) for photons, leading to the suggestion that 'whole body' dosemeters currently designed (figure 6(a)) to assess photon personal dose equivalent at a depth of 10 mm could respond poorly if required to assess Hp(θ, ε). The impacts of this proposed change for the PHE TLD are shown in figure 6(b) for monoenergetic x-ray and radionuclide exposures from θ = 0°, 30°, 60°, ROT and ISO. Clearly, the TLD over-responds significantly for all low-energy exposures, and is also an under-estimator of risk from ROT exposures at intermediate energies. Re-scaling or recalibration may help to improve the overall response characteristics slightly, though the benefits of this are less than have been observed elsewhere following the application of similar analyses to PADC neutron personal dosemeters (Tanner et al 2018) or neutron survey instruments (Eakins et al 2018). In general, however, the photon Hp(θ, ε) response characteristics of the current design of PHE TLD would fail to comply with the IEC performance recommendations (IEC 2012), though it is noted that those are defined in terms of Hp(10, θ, ε) so may not strictly be appropriate.
The above observations imply that the PHE TLD would need to be redesigned to better match the Hp(θ, ε) requirements for photons. To that end, a simple adaption has been suggested, featuring a composite filter comprising 1.5 mm thick aluminium and 2.8 mm thick PTFE. In principle, the response characteristics of the redesigned dosemeter could potentially be refined further, for example by: considering other materials in the composite; by considering combinations of three or more materials in the composite; or by relaxing the condition that the total thickness must remain ≤4.3 mm. All of these possibilities could be investigated as part of a full redesign campaign for the PHE TLD, with the latter demanding that the PP holder would also have to be modified and so would presumably prove to be the most expensive option. Such a campaign would, of course, only commence if the proposed dose quantity were accepted and when the standards describing calibration protocols and performance requirements had been revised, and would presumably progress in parallel with other dosimetry services likewise choosing to restructure their dosemeter designs at that stage. Nevertheless, it is encouraging that the simple modification suggested here, which could be used within the holder of the present design of dosemeter, provides acceptable Hp(θ, ε) relative response characteristics across the photon energy and angle range of interest (figure 7).
The proposed quantity personal absorbed dose in local skin is sufficiently similar to Hp(0.07, θ, ε) for electrons above ∼150 keV (figure 3) to suggest that the TLD's 'skin element' would be relatively unaffected by the proposal if required to respond in terms of Dp skin(θ, ε); electrons with energies <150 keV are of less concern outside of extremity dosimetry. However, problems arise (figure 8) when considering the electron Hp(θ, ε) response of the TLD because, whilst the Hp(10, θ, ε)/Φ conversion coefficients are essentially zero for beta and low-energy electron sources, Hp(θ, ε)/Φ is non-zero even at extremely low energies due to the contribution to effective dose from skin doses. Of course, it is important to monitor the stochastic risk to skin as well as the risk from tissue reactions, so the inclusion of beta doses in Hp(θ, ε) is necessary if Hp(0.07, θ, ε) were to be 'replaced' by Dp skin by ICRU. This implies that a TLD ought to respond to electrons of all energies. But, it may prove very difficult to measure electron Hp(θ, ε) doses across the full energy range using a single detector element, due to fundamental differences in their penetrating powers and hence their filtration requirements.
On the other hand, it might be remarked that whole body exposures to high-intensity electron sources are not very common and low-energy electrons are unlikely to contribute much dose to an individual's overall burden. Moreover, the skin dose will be limiting when it is >25× larger than personal dose, which is the case for electron energies between 60 keV and 1.5 MeV (figure 4); lower energy electrons are too short-ranged to be of much concern for whole body dosimetry. The need to assess the stochastic risk to the whole body from electron exposures, as quantified by Hp(θ, ε), might therefore only be limiting in relatively rare circumstances. Consequently, the above criticisms may primarily be ones of principle, rather than problems for practical individual monitoring in realistic workplace fields. It might therefore be possible to disregard them in many cases, though guidance here from ISO and IEC would obviously be necessary before proceeding. Arguably, however, such a workaround would be intrinsically unsatisfactory: if personal dose quantities are defined at a given energy, and conversion coefficient data are published by ICRU, it seems reasonable to presume that personal dosemeters ought to be both required and able to measure them. The relevance of the quantity to operational protection is otherwise questionable.
Conclusions
The current PHE TLD features an uncovered element that is able to measure Hp(0.07, θ, ε) for photons and electrons, and a covered element that is able to measure Hp(10, θ, ε) for photons and electrons, across the whole energy range of interest. The redesigned TLD features an uncovered element that can be used to measure Dp skin(θ, ε) for photons and electrons, and a covered element that can measure Hp(θ, ε) for photons, across the whole energy range. But, neither element of the proposed TLD is able to accurately measure Hp(θ, ε) for electrons across this energy range. Moreover, it may prove difficult to further re-optimise the TLD such that Hp(θ, ε) for electrons could be measured satisfactorily, and potentially impossible for a two-element TLD to be able to simultaneously measure both Hp(θ, ε) and Dp skin(θ, ε) for both photons and electrons: the four sets of energy and particle-dependent requirements are simply too different.
If the proposed quantities were accepted as they currently stand, the above limitation might reduce to the question: what should two-element body dosemeters optimally monitor in mixed photon/electron fields? In the absence of any accepted recommendations, it is unclear at present as to how the PHE dosimetry service may choose to approach this, but potential solutions to explore could be:
- Restricting the permitted use of the redesigned TLD to fields that do not contain primary electron sources;
- Restricting the permitted use of the redesigned TLD to fields in which the primary electron component to Hp(θ, ε) can be neglected, with the concurrent measurement of Dp skin(θ, ε) for electrons by the uncovered element assumed to provide the limiting dose assessment. This may be the case in many realistic workplace fields, with large whole-body exposures to beta sources potentially unlikely in practice, but agreement and recommendations would first be required from legislative bodies on what can and cannot be considered negligible. The redesigned TLD could not reliably be used in fields with electron components between ∼1.5 MeV and ∼15 MeV, for which it is Hp(θ, ε) rather than Dp skin(θ, ε) that is limiting;
- Repurposing the two elements of the TLD, such that the uncovered element provides Hp(θ, ε) assessments for photons and betas but the covered element only provides Hp(θ, ε) for photons. Accurate characterisation of the workplace field would be required to reconstruct doses in such an arrangement, however, so that bespoke calibration corrections could be applied and double-counting of the photon contribution could be avoided; development of advanced dose reconstruction algorithms might also be required. The IEC performance criteria might also need to be amended to cope with the significant energy-dependence of Hp(θ, ε) for betas, with a factor a ∼few over- or under-response perhaps needing to be allowed. In principle, additional calibration factors could also be applied to simultaneously assess Dp skin(θ, ε) with the uncovered element;
- Completely redesigning the TLD. It is conceivable that a fully-metallic or even perforated metal filter might provide both some limited 'transparency' and some limited filtration that allows both betas and photons, of both low and high energies, to be measured by a single element. Such a design is just a speculation at this stage, however, and would be difficult to optimise to avoid a poor energy and angle dependence of response. Alternatively, the Harshaw TLD-700H card employed in the PHE TLD has space for two further sensitive elements. A more effective solution might therefore be to also incorporate these, perhaps covered by alternative filters with different calibrations, to allow different components of the field to be assessed independently; weighted combinations of the results could then potentially provide Dp skin(θ, ε) and Hp(θ, ε) for both photons and electrons. Of course, there would be significant cost implications for such a redesign, both initially to PHE and ongoing to the users of the dosimetry service.
The effectiveness of any re-optimisation campaign would be judged against the performance criteria stipulated for personal dosemeters (IEC 2012). However, it is remarked that those recommendations would, in time, also preferably be updated to better reflect the requirements of the proposed dose quantity. In turn, the accredited dosimetry services who supply personal dosemeters to customers may likewise need to recalibrate or replace their existing systems with the updated versions, in order to better assess the new dose quantity in their respective workplace fields and comply with new recommendations once accepted. The logistics, timescale and financial implications of such a renewal programme would likely vary on a case-by-case basis, but indicate that the potential benefits of moving from Hp(d, θ, ε) to Hp(θ, ε) and Dp skin(θ, ε) in terms of more accurate risk assessments would inevitably be weighed against potential costs.
It may prove to be the case that the new operational quantities open up better options for dosemeter design, either by adjustments to the existing designs or by making more radical redesign feasible. For example, one challenge in designing dosemeters worn at a single location on the front of the body is that they typically under-estimate risk following exposures from the rear. A full redesign project could potentially seek to address this issue as part of its remit, though exactly how this might be achieved is unclear at this stage. Nevertheless, any attempt would at least be a step in the right direction: Hp(10, 180°, ε), for instance, is a somewhat flawed quantity in many cases, being significantly smaller than effective dose PA, but Hp(180°, ε) is numerically equal to E(PA, ε)/Φ. In that vein, it is encouraging that for photons the Hp(θ, ε) relative response of the simple redesigned dosemeter (figure 7) was generally comparable to the Hp(10, θ, ε) relative response of the current dosemeter (figure 6(a)) in terms of its overall performance, whilst Hp(θ, ε)/Φ is clearly a much better estimator of risk than is Hp(10, θ, ε)/Φ, being directly relatable to effective dose. However, whether or not this type of advantage truly warrants the additional complications and complexities associated with updating the operational dose quantity is a cost-benefit analysis for elsewhere.
Ackowldegements
The authors thank Luke Hager of PHE for helpful comments and for performing the folding used to generate the conversion coefficients for beta sources.