Abstract
Many methods have been proposed to accelerate the oxygen reduction and save the dosage of Pt. Here, we report a promising way in fulfilling these purposes by applying substrate strain on the supported Pt monolayer. The compressive strain would modify the geometric and electronic structures of tungsten carbide (WC) substrate, changing the interaction nature between substrate and Pt monolayer and resulting in a downward shift of the d-band center of surface Pt atoms. The activity of Pt monolayer on the compressed WC is further evaluated from the kinetics of the dissociation and protonation of O2. The dissociation barrier of O2 is increased and the hydrogenation barrier of O atom is decreased, indicating that the recovery of the catalytically active sites is accelerated and the deactivation by oxygen poison is alleviated. The present study provides an effective way in tuning the activity of Pt-based catalysts by applying the substrate strain.
Export citation and abstract BibTeX RIS
1. Introduction
The sluggish kinetics of oxygen reduction reaction (ORR) at the cathode of proton exchange membrane fuel cell (PEMFC) is a longstanding obstacle to its commercialization even if the most efficient but very expensive Pt-based catalysts are used [1, 2]. A large number of experimental and theoretical studies have thus focused on accelerating the ORR and reducing the dosage of Pt [3–7]. Some potential methods including alloying with foreign metals [8], decreasing the size of nanoparticles [9], assembling Pt-based core–shell nanostructures with base transition metals in the core [10] are proposed accordingly. Moreover, introducing lattice strain in Pt is also an effective method to enhance the ORR activity [11, 12]. Very recently, Wang et al [13] reported that the lattice strain of Pt can be controlled directly and continuously by electrochemically switching between the charging and discharging status of battery electrodes, and they observed 90% enhancement in ORR activity under compression. However, the dosage of Pt in Wang et al's method is higher compared with the Pt monolayer (ML) loaded on the transition metal carbide nanoparticle (e.g. tungsten carbide (WC)) [14], which also exhibits enhanced activity and resistance to sintering and CO poisoning. As a matter of fact, deposition of Pt ML on different substrates is another promising way in tuning the activity and lowering the dosage of Pt [15–19].
By comparing the kinetics of ORR on the Pt ML deposited on various transition metal substrates, Zhang et al [20] found that the Pt ML on the substrates with smaller lattice constants than Pt exhibits comparable activity to pure Pt. Our early studies also indicated that the Pt ML on transition metal carbide surface exhibits unexpected activity over pure Pt [21]. The compressive strain weakens the adsorption strength of the adsorbates because the d-band center of catalysts moves down in energy, which will accelerate the diffusion and recombination of the intermediates, while the expansive strain exhibits opposite effects [22]. Grabow et al [23] compared the reaction energetics and mechanisms of CO oxidation on Pt(1 1 1) surface with different strains, and found that compressed surfaces favor the associative reactions. The results imply that one can speed up or slow down a certain reaction step to achieve an optimal efficiency by applying suitable strains.
Generally, the lattice strain could be introduced via the epitaxial growth or the pulsed laser deposition of the metal layers on different substrates [24]. When the selected substrates have smaller lattice constants than the supported metal layers, the compressive strain on the supported metal layers is achieved [20, 25]. For the core (alloy)-shell structures, the strain is commonly tuned by changing the compositions of the alloy layer. For example, Adzic's group [10] found that the electrocatalytic activity of Pt monolayer shell is varied with the change in the compositions of the bimetallic IrM (M = Fe, Co, Ni or Cu) cores. By comparing the density functional theory calculations and experimental results, they pointed out that the core should induce sufficient contraction to the Pt shell for improving the activity. However, it is difficult to tune the strain continuously by adopting these methods due to the limit in the appropriately alternative substrates. Here, taking the WC substrate as an example, we report that compression of the substrate may be a feasible method to introduce the continuous strain on the Pt ML. Importantly, this method could simultaneously realize the targets for improving the activity and reducing the dosage of Pt, which are very important to promote the development of the PEMFC. We choose WC as the substrate of Pt ML based on the following considerations: (1) its electronic nature is very similar to Pt [26]; (2) it can stabilize the supported Pt [27]; (3) the activity and the resistance to CO poisoning of Pt can be improved by WC substrate [21, 28].
In ther present study, the effects of the substrate strain on the electronic structure and catalytic activity of the supported Pt ML were comparatively investigated. We indeed found that the Pt ML is compressed with the constriction of WC substrate. In the meantime, the d-band center of the Pt ML moves down and the O removal rate by the hydrogenation process is increased. We expect that this study could provide a theoretical guidance to improve the activity of the catalysts and reduce the usage of Pt.
2. Methods and models
The spin-unrestricted calculations were performed using a projector augmented wave (PAW) method [29] as implemented in the Vienna Ab initio simulation package (VASP) [30]. The exchange correlation potential was described using the generalized gradient approximation of PW91 functional [31], which is well suited for studying the geometric and electronic features of the concerned systems based on previous results [27, 32, 33]. The convergence criteria for total energy and forces were set as 10−6 eV and 0.02 eV Å−1, respectively. The k-points were sampled in the Brillouin-zone integration with a 7 × 7 × 7 k-point mesh generated by adopting the Monkhorst–Pack scheme for the optimization of the lattice constants, and a 3 × 3 × 1 k-point mesh for the geometry optimization of the slab model and the following transition states determination, while a denser mesh of 11 × 11 × 1 was used for calculating the electronic properties. The climbing image nudged elastic band method (CI-NEB) [34] was employed to investigate the transition states and minimum energy paths. The spring force between adjacent images was set as 5.0 eV Å−1. The energy barriers were calculated using the initial state as a reference. First-principles molecular dynamics (MD) was performed with a single K point at Γ to sample the Brillouin zone to verify the dynamic stability. A time step of 1 fs was used and the temperature was controlled at 350 K in a canonical ensemble which was simulated using the algorithm of Noséz [35]. The zero point energy and the solvent corrections were excluded here and the effects of these can be found in literatures [28, 36].
The model used here (insertion in figure 1) is in consistent with our previous studies [27, 28]. A (3 × 3) WC(0 0 0 1) surface with W termination was selected as the substrate of the Pt ML. The supercells were modeled as periodically repeating slabs with three WC bilayers and a vacuum layer of 15 Å to eliminate the slab–slab interaction. During optimization, the most bottom four atomic layers of the slabs were constrained at their bulk positions to mimic bulk characteristics, while the remaining layers including the Pt ML and the subsequent adsorbates were allowed to relax freely.
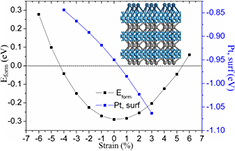
Figure 1. The formation energies of WC bulk with different strains (black curve), and the surface energies of Pt monolayer on WC(0 0 0 1) surface with different substrate strains (blue curve). The insertion is the model used in the present study.
Download figure:
Standard image High-resolution image3. Results and discussions
The substrate strain is achieved by changing the equilibrium lattice constant parallel to the WC(0 0 0 1) surface, and defined as

where a and a0 are the lattice parameter of the unit cell with and without strain, respectively. A similar approach has been adopted to investigate the effects of strain on the dissociation of CO on Ru(0 0 0 1) surface [37]. The strain limits on WC bulk were determined by the formation energy (Eform) using equation:
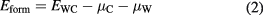
in which EWC is the energy of WC bulk with strain; μC and μW are the chemical potentials of carbon and tungsten atom, respectively, defined as the atomic energies of perfect graphene and W bulk. It is found from figure 1 (black curve) that a large range of strain from about −4% to 5% is endurable for WC since the formation energies are lower than 0 eV. To verify whether the Pt ML can be formed on the strained WC(0 0 0 1) and quantify its thermodynamic stability, the surface energy is calculated based on the equation [38]:
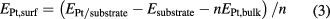
where the EPt/substrate is the total energy of Pt ML on WC(0 0 0 1) surface with substrate compression (PtML/c-WC) or tension; Esubstrate is the total energy of the compressed or stretched substrate; n is the number of Pt atoms, and EPt,bulk is the energy per Pt atom in Pt bulk. As shown in figure 1 (blue curve), the expansive strain lowers the surface energy, while the compressive strain enlarges the surface energy. In the allowable range of strain, all the surface energies of the PtML/c-WC models are negative, indicating the thermodynamic stability of Pt ML on the strained WC substrate. The Pt ML is stretched by about 4% on the unstrained WC(0 0 0 1) surface with W termination, i.e. the scission of O–O bond should be easier on Pt ML supported on unstrained WC(0 0 0 1) (PtML/un-WC) than on pure Pt, but the protonation reaction is slower on PtML/un-WC than on pure Pt [27]. Previous studies showed that PtML/un-WC has advantages in stability and the resistance to CO poisoning, but is easy to be poisoned by adsorbed O due to the intensive interaction between them [27, 28]. It is thus necessary to compress the Pt ML for weakening its interaction with the adsorbed O and facilitating the recovery of the active sites. Consequently, we focus mainly on the compression of the WC substrate in the following study. Ab initial molecular dynamic simulations are then performed to further corroborate the dynamical stability of PtML/c-WC (see the supplementary material (stacks.iop.org/JPhysD/50/435501/mmedia)).
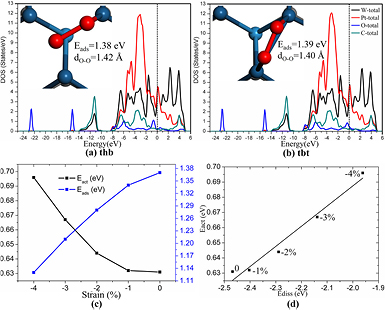
The adsorption and dissociation of O2 on PtML/WC with and without the substrate strain are investigated to evaluate the effect of substrate compression on the activity of Pt ML. The adsorption of O2 is the prerequisite of the following reactions and the O2 dissociation is a representative O–O bond scission step. For O2 on PtML/un-WC, two competitive adsorption configurations are found. One is that the O2 locates at the hollow site with a Pt atom directly beneath it (labeled as thb configuration in figure 2(a)). The obtained O–O bond length and adsorption energy (Eads) are 1.42 Å and 1.38 eV, respectively. Here, the adsorption energy is defined as the energy difference of the total energy of isolated substrate and adsorbate from the adsorbed system. The other is that the O2 adsorbs at the bridge site of Pt–Pt bond (labeled as tbt configuration in figure 2(b)) with the O–O bond length of 1.40 Å and an adsorption energy of 1.39 eV. The density of states (DOS) is studied to further understand the interaction characterizations between O2 and PtML/un-WC. As shown in figures 2(a) and (b), the tbt configuration has similar DOS features to the thb configuration. The lowest two peaks are contributed by the O-s states, the peaks located in the energy range from −14 to −10 eV come from the s states of W and C, and the main peaks in the energy range from −8 to 6 eV are the hybridized states of O-p, C-p, W-d and Pt-d. Nonetheless, the DOS peaks of O2 around the Fermi energy between the two adsorption configurations are different. The 2π* states of O2 are partially filled for the tbt configuration, while they are fully filled for the thb configuration, resulting in the larger O–O bond length and weaker O–O bond strength for the thb configuration comparing to the tbt configuration. Consequently, the dissociation of O2 from thb configuration may be easier than that from the tbt configuration. The geometric and electronic structures of O2 adsorbed on the PtML/c-WC are found to be almost the same as those on PtML/un-WC; we have summarized these results in the supplementary material.
Figure 2. The DOS of O2 adsorbed on the PtML/un-WC with thb (a) and tbt (b) configurations. The corresponding adsorption parameters are displayed. Shown also in the figures are the adsorption energies (blue curve) and dissociation barriers (black curve) of O2 on the Pt ML as a function of the substrate compression (c), and the BEP relation between the Eact and Ediss (d), where Ediss is the dissociation adsorption energy of O2 defined as: Ediss = 2EO-substarte − 2Esubstrate − EO2.
Download figure:
Standard image High-resolution imageFrom figure 2(c), accompanied with the compression of the substrate from 0 to 4%, the adsorption energies of O2 (Eads, blue curve) decrease gradually from about 1.38 to 1.13 eV, in line with the experimental observations that the compressive strain on the Pt films weaken the interactions between Pt and the adsorbates [39]. But the energy barriers for O2 dissociation (Eact, black curve) increase from about 0.63 to 0.70 eV, in agreement with the Brønsted–Evans–Polanyi (BEP) relationship [40] between the dissociation adsorption energy of oxygen (Ediss) and Eact, i.e. the stronger adsorption (lower of the Ediss), the smaller of the Eact (figure 2(d)). These results indicate that the compressed WC substrates weaken the interaction of O2 with Pt ML and slightly suppress the dissociation of O2. Generally, two factors modulate the activity of the Pt ML on compressed WC. One is the electronic interaction between the WC substrate and Pt ML (ligand effect) and the other is the lattice strain induced by the lattice mismatch between them (surface strain effect). The two effects simultaneously impact the catalytic activity and can hardly be isolated [41]. However, the ligand effect is overwhelmingly strong in the ML situation [42, 43]. We thus focus mainly on the electronic interactions.
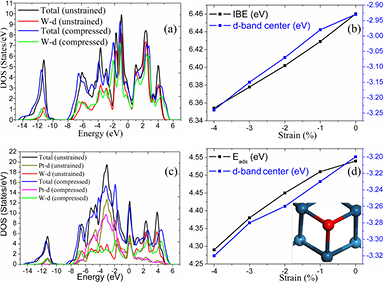
As shown in figure 3(a), the peaks of the DOS of the compressed WC are usually lower and broader than those of the unstrained WC, because the compressive stress increases orbital overlap between the atoms, broadening their bands. Especially, the valley at the −2 eV below the Fermi energy is filled due to the compression and the d-band center of surface W atoms moves far away from the Fermi level (blue curve in figure 3(b)), leading to weakening of the interaction between the compressed WC substrate and Pt ML, characterized by the integral binding energy (IBE) [44] with the definition of
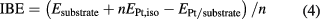
where the EPt,iso and n are the energy of single Pt atom and the number of Pt atoms in the system, respectively. From figure 3(b) (black curve), one indeed can see that the value of IBE becomes smaller and smaller with the increasing in substrate compression. Although the reduced adsorption of the compressed WC to Pt ML is disadvantageous to its stability, the Pt ML can still be formed and stable based on the aforementioned results. Moreover, the DOS peaks of the supported Pt atoms on the compressed WC substrates become lower and broader compared to those on the unstrained substrate (figure 3(c)), and the d-band center of the surface Pt atoms moves consequently farther away from the Fermi level (blue curve in figure 3(d)).
Figure 3. The DOS of WC(0 0 0 1) with and without compression (a), the d-band center of surface W atoms (blue curve) and the IBE (black curve) against to the compressive stress (b), the DOS of Pt monolayer on WC(0 0 0 1) surface with and without the substrate compression (c) and the d-band center of the Pt monolayer (blue curve) and the adsorption energy of O atom (Eads, black curve) on PtML/WC as a function of the WC substrate compression (d).
Download figure:
Standard image High-resolution imageOxygen atom always prefers to be adsorbed at the hollow site with no atoms beneath it (insertion in figure 3(d)), but the adsorption energy of O decreases with increasing in compression, which is considered to be beneficial to improving the performance of Pt ML towards ORR. On one hand, the durability of catalysts may be enhanced due to the reduced probability in the corrosion of the substrate by the adsorbed O atoms. On the other hand, the OH formation which was previously suggested as the possible rate-limiting step of ORR on the PtML/un-WC [27] becomes easier because the reduced interaction between O and PtML/c-WC accelerates its diffusion and hydrogenation, alleviating the catalytic deactivation.
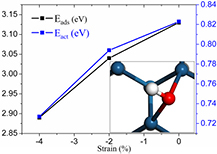
The most stable adsorption configuration of OH is depicted in figure 4. The energy barrier of the hydrogenation of O to form OH is decreased from about 0.82 eV to 0.73 eV with the compression of the WC substrate from 0 to 4%. Meanwhile, the adsorption energy of OH is reduced to about 2.88 eV from 3.12 eV, indicating that the hydrogenation of O and OH to form H2O on Pt ML is easier on compressed WC substrate than on the unstrained substrate [45]. The fast hydrogenation of O and OH will reduce their coverage on the compressed Pt ML, agreeing with the experimental findings that the high ORR catalytic activity is associated with a reduced OH coverage [46]. Similar conclusion was drawn by Rhen and McKeown [39], who studied the oxidation of methanol on strained Pt films. They observed that the rate of methanol oxidation is correlated to the surface coverage of methanol which is depending on the level of compressive strain. Moreover, we find that a bigger extent of compression will lead to the higher activity of Pt ML towards ORR, which is also in line with the recent experimental suggestions that the Pt layer should be compressed sufficiently to possess the high activity [10].
Figure 4. The dependencies of the formation barriers (blue curve) and adsorption energies (black curve) of OH on the substrate compression. The insertion is the most stable configuration of OH on Pt ML supported on WC(0 0 0 1).
Download figure:
Standard image High-resolution imageBased on the kinetic results, the easier the dissociation of O2, the harder the formation of OH, and vice versa, in good agreement with the previous studies [3, 47]. Thus, a good catalyst for ORR should have the barrier of O2 dissociation close or equal to that of OH formation. The dissociation of O2 and the formation of OH on Pt ML supported on Pd(1 1 1) (PtML/Pd(1 1 1)) have almost the same barrier of 0.79 eV. Zhang and coworkers [20] accordingly suggested the high activity of PtML/Pd(1 1 1) towards ORR. Here, we found that the barrier for O2 dissociation (0.70 eV) is close to that for OH formation (0.73 eV) on the Pt ML supported on WC(0 0 0 1) with 4% compression, the barriers are also comparable to those on PtML/Pd(1 1 1) (~0.79 eV), indicating a similar high activity. The advantages here lie in the higher thermal stability [14] and lower price of the WC(0 0 0 1) substrate as compared with the Pd(1 1 1). Moreover, different with the methods of loading Pt layer on various substrates [20, 25] or changing the composition of core layer of the core–shell structures [10, 48, 49], the compression of the substrate could achieve the continuous tunability in strain applied on the supported Pt ML. Importantly, the demands of enhanced activity and reduced dosage of Pt could be realized simultaneously using this method. We anticipate that the present study could provide a theoretical guidance on designing the highly active catalysts with the reduced dosage of Pt by applying the substrate strain.
4. Conclusions
In conclusion, the effects of the compression of WC substrate on the activity of Pt monolayer (ML) towards oxygen reduction reaction were investigated using first-principle calculations. It is found that the compressive stress would tune the geometric and electronic structure of the WC substrate as well as the supported Pt ML, leading to the d-band center of the surface Pt atoms shifting downward from the Fermi level. Consequently, the interaction between the Pt ML and the adsorbates including O2, O, and OH would be reduced; while the dissociation barrier of O2 is slightly enlarged, the barriers of the hydrogenation of O to form OH and finally to form H2O are decreased. These changes would be beneficial to accelerating the recovery of the catalytically active sites and alleviating the deactivation by oxygen poison, thus improving the performance of Pt-based catalysts towards ORR. The present study provides an effective way in tuning the activity and reducing the dosage of Pt towards oxygen reduction.
Acknowledgments
This work is supported by the National Natural Science Foundation of China under Project Number 11474086 and the Early Career Scheme of RGC under Project Number 27202516. The simulations are performed on resources provided by the High Performance Computing Center of Henan Normal University and those provided by College of Physics and Materials Science in Henan Normal University.