Abstract
In this work, we report an all-solution route to produce semi-transparent high efficiency perovskite solar cells (PSCs). Instead of an energy-consuming vacuum process with metal deposition, the top electrode is simply deposited by spray-coating silver nanowires (AgNWs) under room temperature using fabrication conditions and solvents that do not damage or dissolve the underlying PSC. The as-fabricated semi-transparent perovskite solar cell shows a photovoltaic output with dual side illuminations due to the transparency of the AgNWs. With a back cover electrode, the open circuit voltage increases significantly from 1.01 to 1.16 V, yielding high power conversion efficiency from 7.98 to 10.64%.
Export citation and abstract BibTeX RIS
1. Introduction
Methylammonium lead halide and mixed halides CH3NH3PbX3 (X = Cl, Br, I) possessing the perovskite structure, are an emerging light-absorbing material with features such as a large light-absorption coefficient, direct band gap [1], high carrier mobility and long carrier diffusion length [2, 3]. The continuous and fast progress in the research field related to perovskite solar cells (PSCs) [1, 4–9] has already established them as a serious contender to those traditional commercialized solar cells such as silicon-based solar panels. Besides their high efficiency, the most attractive feature of perovskite-based solar cells is that they can be fabricated with low-cost solution processing [10]. Moreover, in the emerging field of transparent architectures in civil technology such as windows, roof tops, greenhouses and other fashion elements, semi-transparent perovskite solar cells have also been receiving growing attention. Meanwhile, the band gap of the perovskite absorber is higher than the commercial ones such as silicon, which makes it viable to make a semi-transparent PSC as the top cell in tandem solar cells.
However, the top electrode of the perovskite solar cell is usually fabricated by high vacuum thermal evaporation of an opaque noble metal, which is relatively expensive and energy consuming. In order to reduce the cost of this vacuum process, some progress has already been made on the low-cost electrode preparation [11–13]. For example, highly efficient and long-term stable perovskite solar cells have been proposed by Mei et al through the printing of carbon black/graphite composite material as the top electrode [13]. However, the carbon black/graphite layer is rather thick (tens of micrometers) for the achievement of the desired sheet resistance, which makes the device non-transparent.
Numerous reports were found on semi-transparent perovskite solar cells, but most of these devices employed thin metal films (Al, Ag, Au) as top electrodes, which were fabricated based on energy-intensive evaporation processes [14–17]. Semi-transparent perovskite solar cells using solution-processed material such as carbon nanotubes [18], transparent conducting adhesive [19] and silver nanowires [20] have also been reported recently. However, the transfer and lamination procedures involved in these fabrication methods increased the complexity of the device fabrication, which is incompatible with large-scale, continuous roll-to-roll manufacturing.
Solution-processed silver nanowires (AgNWs), which are easily fabricated, have been shown to produce uniform, highly conductive and transparent films with great potential to make transparent devices [21]. Efficient and bendable organic solar cells (OSCs) were fabricated using solution-processed AgNWs electrodes with an efficiency of 5.8% [22]. Semi-transparent dye-sensitive solar cells (DSSCs) fabricated using AgNWs/PEDOT:PSS transparent electrodes showed power conversion efficiencies of 3.6% [23]. Recently, spray-coated AgNWs used as the top electrode of PSCs with inverted structure have been studied, where ZnO was used as the interlayer to improve the ohmic contact between the AgNWs and the PCBM with an efficiency of 8.49% achieved [24]. A novel structure of fiber-shaped flexible PSCs has also been introduced using AgNWs as the top electrode with a 3.85% efficiency [25].
Here, we introduce a simple spray-coating technique for efficient semi-transparent PSCs with conventional structure. With careful control of the spray-coating conditions, the top AgNWs electrode can be deposited in atmosphere under room temperature without damaging or dissolving the underlying PSC layers. Compared to the inverted structure, the conventional structure has a better energy level match between the underlayer (spiro-MeOTAD) and the AgNWs as shown in figure 1(b), which results in better ohmic contact and higher Voc without any interlayer. With the back cover electrode, Voc up to 1.16 V and an efficiency of over 10% have been achieved. Besides the performance improvement, the back cover electrode could also enhance the stability with proper encapsulation. This simple solution-processed AgNWs electrode will not only reduce the high production cost and complexity of thermal evaporation, but have the benefit of making a semi-transparent device which can be integrated into building windows or be made as the top cell on tandem solar cells [23]. Our progress will pave the way for the realization of large-area all-solution processed semi-transparent PSCs.
Figure 1. (a) Schematic architecture of the perovskite solar cell device. (b) Energy band diagram of the perovskite solar cell. (c) Cross-sectional SEM image of the perovskite solar cell device. (d) Top-view SEM image of the perovskite film. (e) Top-view SEM image of the AgNWs film.
Download figure:
Standard image High-resolution image2. Experimental details
2.1. Perovskite device fabrication
Fluorine-doped tin oxide (FTO) glasses were subsequently cleaned in deionized water, acetone and ethanol, and treated with a UV Ozone (UVO) cleaner for 20 min. The titanium dioxide (TiO2) blocking layer (bl-TiO2) was spin-coated on the cleaned FTO substrate at 2000 rpm for 20 s using 0.15 M titanium diisopropoxide bis(acetylacetonate) in 1-butanol solution and then heated at 125 °C for 5 min. After cooling to room temperature, the TiO2 paste(0.1 g ml−1 in ethanol) was spin-coated at 2000 rpm for 10 s. After drying at 100 °C for 5 min, the film was annealed at 550 °C for 30 min. The mesoporous TiO2 film (mp-TiO2) was immersed in 0.02 M aqueous TiCl4 solution at 90 °C for 10 min. After washing with deionized water and drying, the film was heated at 500 °C for 30 min. CH3NH3PbI3 was formed by using a two-step spin-coating procedure according to the literature procedures [26]. A PbI2 solution was prepared by dissolving 460 mg PbI2 in 1 ml N,N-dimethylformamide (DMF) by heating it at 70 °C. When fully dissolved, the PbI2 solution was spin-coated onto the mesoporous TiO2 film and then dried at 40 °C for 3 min and 100 °C for 5 min, respectively. After cooling to room temperature, the CH3NH3I solution (8 mg ml−1 in 2-propanol) was loaded onto the PbI2-coated substrate for 20 s, then spun at 4000 rpm for 20 s and dried at 100 °C for 5 min. A 2,2',7,7'-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene (spiro-MeOTAD) solution was spin-coated onto the CH3NH3PbI3 perovskite layer at 4000 rpm for 30 s. The spiro-MeOTAD solution was prepared by dissolving 72.3 mg of spiro-MeOTAD in 1 ml of chlorobenzene, to which 29 μl of 4-tert-butyl pyridine (TBP) and 18 μl of lithium bis(trifluoromethanesulfonyl)imide (Li-TFSI) solution (520 mg LI-TSFI in 1 ml acetonitrile) were added. The devices were kept in a dry container until the AgNWs were spray-coated.
2.2. AgNWs electrode fabrication
Silver nanowires were provided by Cold Stones Tech. in a 5 mg ml−1 isopropanol (IPA) solution. Different volumes (200, 400, 600 μl) of this solution were loaded into a spray gun (Gunpiece) and sprayed directly onto the perovskite devices. Nitrogen gas at 0.15 MPa was delivered to the nozzle. The nozzle was positioned 55 mm above a motorized, computer-controlled X-Y stage, onto which the perovskite devices were taped. The spray deposition was carried out intermittently. Each spray would last for 5 s, and the intermittent time was 10 s. For each sample, the spray process was operated in atmosphere under room temperature within 20 min. The samples were kept in a dry container until the test.
2.3. Device characterization
The characterization of the as-fabricated PSCs was carried out with a solar simulator (Sun 2000, Abet Technologies, Milford, CT, USA) under AM 1.5G illumination, which was determined by a calibrated crystalline Si-cell. The current density–voltage (J–V) characteristics were collected with a Keithley4200 semiconductor characterization system (Keithley, Cleveland, OH, USA). The active area of the PSCs was 0.15 cm2. The transmittance and absorption of the semi-transparent devices were measured using a UV–vis-NIR spectrometer (UV-3600, Shimadzu, Japan). Scanning electron microscope (SEM) images of the electrodes and solar cell devices were measured using a field scanning electron microscope (Nova NanoSEM230, FEI, USA).
3. Results and discussion
A schematic of the solution-processed perovskite solar cell in this study with the structure of FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/spiro-MeOTAD/AgNWs is shown in figure 1(a) and the relevant energy band diagram is shown in figure 1(b). Figure 1(c) shows the cross-sectional SEM image of the whole device, from which we can see a well-defined layer-by-layer structure with the AgNWs lying on top of the device. This clear structure indicates full device performance without detrimental effects from shunting or short-circuiting. Among the several layers of the solar cell, perovskite acts as the light absorber, the morphology of which is important to the device performance. With a two-step spin-coating procedure [26], a uniform and fully covered perovskite film was formed as shown in figure 1(d). The absorption and transmission spectra of the perovskite film are shown in figure 2, which indicate good absorption in the visible region obtained by the perovskite film, while semi-transparency could be simultaneously realized.
Figure 2. Absorption and transmission spectra of perovskite film and devices with different thickness of AgNWs (illuminated from FTO side).
Download figure:
Standard image High-resolution imageTo fabricate the transparent top electrode, here we deposited the AgNWs out of isopropanol (IPA) solution directly onto the hole transport material (HTM) using a spray gun with a nitrogen-gas-driven atomizer nozzle. As shown in figure 1(e), the spray-coated AgNWs formed a uniform and well-dispersed film on the HTM. Solvent damage to the underlying layers can be avoided during the AgNWs deposition by carefully choosing the key deposition parameters to minimize the amount of liquid solvent that reaches the device surface [23]. IPA was chosen as the solvent of the AgNWs for its low boiling point, further reducing the amount of solvent reaching the device surface. The AgNWs film thickness (and thus transmission and conductivity) is easily controlled by varying the volume of the AgNWs solution used.
To compare the impact of the AgNWs film thickness on the device performance, we fabricated the device electrode with varying volumes (200, 400, 600 μl) of AgNWs solution (5 mg ml−1 in IPA). The thicknesses of the deposited AgNWs films are 200, 380 and 750 nm, respectively, which were determined by cross-sectional SEM testing. Figure 2 shows the absorption and transmission spectra of the perovskite devices fabricated under various conditions measured from 400 to 800 nm. It is clearly observed that without the AgNWs electrode, the device shows a transmission of about 67% at 800 nm. With increasing the thickness of the AgNWs (from 200 to 750 nm), the transmission decreases reasonably from about 55 to 35% at 800 nm. The absorption of the device increases with thicker AgNWs film, which results in higher current density (Jsc) being generated. The J–V curve and performance metrics of the corresponding perovskite solar cells are shown in figure 3 and table 1, respectively. Due to the transparency of the AgNWs layer, the perovskite solar cells can be illuminated from both the FTO and AgNWs sides. When illuminated from the FTO side, the current density (Jsc) is increased with the AgNWs film thickness, which is mainly because the thicker and denser AgNWs film helps to collect more charges transported from the HTM layer. Meanwhile, the absorption of the perovskite absorber increases (as shown in figure 2) as the thicker and denser AgNWs film reflects more sunlight into it. The current density of the device with 750 nm AgNWs reaches 20.8 mA cm−2, which is comparable with that of a relevant opaque perovskite device. To verify the accuracy of the measured current density, the incident-photon-to-current conversion efficiency (IPCE) for the relevant perovskite solar cell is presented in figure 4(b). By contrast, Jsc is lowered when the devices are illuminated from the AgNWs side. This is due to the relatively weaker transmission of the AgNWs and spiro-MeOTAD than the FTO and TiO2 [23]. As a result, the device with 750 nm AgNWs shows the best result with an efficiency of 4.86% when illuminated from the AgNWs side and an efficiency of up to 7.98% when illuminated from the FTO side.
Figure 3. J–V curve of the perovskite devices with different film thickness of the AgNWs illuminated from both sides (FTO and AgNWs sides).
Download figure:
Standard image High-resolution imageTable 1. Performance metrics of perovskite devices with different film thickness of the AgNWs illuminated from both sides of the devices (FTO and AgNWs sides).
| AgNWs thickness | Illumination direction | Jsc (mA cm−2) | Voc (V) | FF (%) | Eff (%) |
|---|---|---|---|---|---|
| 200 nm | AgNWs side | 7.6 | 0.96 | 30.8 | 2.23 |
| FTO side | 14.8 | 0.98 | 31.5 | 4.56 | |
| 390 nm | AgNWs side | 11.8 | 0.95 | 38.6 | 4.31 |
| FTO side | 19.6 | 0.97 | 34.1 | 6.47 | |
| 750 nm | AgNWs side | 13.2 | 0.96 | 38.6 | 4.86 |
| FTO side | 20.8 | 1.01 | 38.1 | 7.98 |
Figure 4. (a) J–V curve of the perovskite device (with 750 nm AgNWs) with and without a back cover electrode (illuminated from FTO side); (b) IPCE of perovskite device (with 750 nm AgNWs).
Download figure:
Standard image High-resolution imageFurther investigation of the cross-sectional SEM image of the AgNWs layer on the device shows that the as-spray-coated silver nanowires are not dense enough, which may influence the carrier transport of the device, as shown in figure 1(c). In view of this fact, we further enhance the performance of the device (with 750 nm AgNWs) by adding an AgNWs-deposited glass as the back cover to the AgNWs electrode with proper pressure to enhance the contact between the AgNWs and the HTM as well as the contact between the AgNWs themselves. After adding the back cover, a remarkable improvement is achieved with Voc and FF increased from 1.01 V and 38.1% to 1.16 V and 43.7%, respectively. As a result, the efficiency is increased significantly from 7.98 to 10.64% when illuminated from the FTO side. The J–V curve and performance metrics of the perovskite device with and without a back cover electrode are shown in figure 4 and table 2, respectively. The series (Rs) and shunt resistances (Rsh) in table 2 were calculated from the slope of the J–V curve close to 2 and 0 V, respectively. From table 2, we can find that the series resistance (Rs) decreases and the shunt resistance (Rsh) increases after adding the back cover electrode. The improvement can be explained by the reduced contact resistance and carrier recombination at the interfaces between the HTM and the AgNWs as well as within the AgNWs themselves for the better ohmic contact [23, 24]. This improvement leads to considerable enhancement of both the Voc and FF, which results in a significant increase in efficiency by more than 30%.
Table 2. Performance metrics of the perovskite device (with 750 nm AgNWs) with and without a back cover electrode (illuminated from the FTO side).
| Test condition | Jsc (mA cm−2) | Voc (V) | FF (%) | Eff (%) | Rs 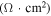
|
Rsh 
|
|---|---|---|---|---|---|---|
| Without back cover | 20.8 | 1.01 | 38.1 | 7.98 | 162 | 239 |
| With back cover | 21.0 | 1.16 | 43.7 | 10.64 | 35 | 10673 |
4. Conclusion
We have developed a solution process by simply spray-coating AgNWs as a top electrode to fabricate a semi-transparent perovskite solar cell. The Jsc is increased with the thickness of the AgNWs for better charge collection and light absorption. The device shows the power conversion efficiency of 4.86 and 7.98% when illuminated from the AgNWs and FTO sides, respectively. Proper back cover contact will further increase the Voc significantly, resulting in a high Voc of 1.16 V and a cell efficiency up to 10.64%. This simple spray-coating technique will effectively reduce the production cost and promote the commercial application of this kind of semi-transparent clean-energy device to building windows and tandem solar cells.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (61377027), the Natural Science Foundation of Fujian Province (2013J01233) and the Education Department Foundation of Fujian Province (JA15076).





