Abstract
Transparent and flexible energy storage devices have garnered great interest due to their suitability for display, sensor and photovoltaic applications. In this paper, we report the application of aerosol synthesized and dry deposited single-walled carbon nanotube (SWCNT) thin films as electrodes for an electrochemical double-layer capacitor (EDLC). SWCNT films exhibit extremely large specific capacitance (178 F g−1 or 552 μF cm−2), high optical transparency (92%) and stability for 10 000 charge/discharge cycles. A transparent and flexible EDLC prototype is constructed with a polyethylene casing and a gel electrolyte.
Export citation and abstract BibTeX RIS
1. Introduction
Electrochemical double-layer capacitors (EDLCs) are supercapacitors based on high surface area carbon, where the charge is stored in the electrochemical double-layer between the electrolyte and the carbon electrode. They can store several times more charge per volume or mass than traditional electrolytic capacitors, their charge/discharge times are fast and they are very stable due to a lack of chemical reactions in the cell [1]. Recently, interest in making transparent and flexible EDLCs has risen due to several applications in displays, touch sensors, photovoltaics, organic light emitting diodes (OLEDs), etc, which hold promise to change the way we use electronics. Carbon nanotubes and other carbon materials can be used to fabricate electrodes for these novel transparent and flexible EDLCs (table 1) [2–15]. However, to achieve high optical transparency one should use ultrathin films, which limits the conductivity of the electrodes and sets a requirement for a very high mass specific capacitance to achieve adequate performance. In this respect, single-walled carbon nanotubes (SWCNTs) are an ideal material for transparent and flexible EDLCs due to their excellent electric conductivity and specific surface area.
Table 1. Literature review of flexible and transparent supercapacitors based on carbon nanomaterials. Specific capacitances (Csp) are normalized to one electrode according to equations (2) and (3) from the values reported in the references.
| Material | Cell type | Transparency @ 550 nm (%) | Csp F g−1 | Csp μF cm−2 | Reference |
|---|---|---|---|---|---|
| SWCNT | test cell | 92 | 178 | 552 | This work |
| CNF-RGO | prototype | 30 | 1730 | [2] | |
| CNT | prototype | 75 | 29.2 | [3] | |
| CNT | 3-electrode | N/A | 60 | [4] | |
| graphene | prototype | 57 | 7.6 | 5.8 | [5] |
| graphene | prototype | 84 | 8.4 | [6] | |
| graphene | prototype | 64 | 24.8 | [7] | |
| graphene | prototype | 73 | 17.3 | 5.33 | [8] |
| graphene | 3-electrode | 70 | 135 | [9] | |
| MWCNT | prototype | 75 | 100 | [10] | |
| MWCNT | prototype | 70 | 6.38 | [11] | |
| nanocups | prototype | 71 | 818 | [12] | |
| SWCNT | 3-electrode | 54 | 12.9 | [13] | |
| SWCNT | prototype | 60 | 90 | 584 | [14] |
| SWCNT | prototype | 58 | 136.8 | [15] |
SWCNTs have many unique properties, which are advantageous for a wide variety of applications including EDLCs. They have exceptionally high Young's modulus of elasticity and tensile strength, and are the strongest known material [16, 17]. The porosity and specific surface area of SWCNT films are very large and they possess high transparency and flexibility. SWCNTs can withstand extremely high currents (up to 109 A cm−2) making them an ideal replacement for copper and aluminum in fast integrated charge/discharge circuits. Due to these properties SWCNT films are thought to be excellent replacements for traditional transparent electrodes, like indium-tin oxide (ITO), which suffers from several serious drawbacks including limited flexibility and chemical resistance, rare raw material, and high refraction and haze. SWCNT networks show improved performance and reduced fabrication cost compared to ITO and organic materials that have been studied as low-cost alternatives [18].
Current techniques for SWCNT thin film preparation are restricted in various ways. In substrate chemical vapor deposition (CVD) the high temperature of SWCNT growth limits the choice of substrates. A solution-based method is a time and resource consuming process with multiple steps including purification and dispersion of SWCNT, which will produce films with defected SWCNTs due to ultrasonic treatment. Therefore, an aerosol CVD process was recently combined with SWCNT dry deposition to solve these problems [19, 20]. SWCNTs grown by ferrocene decomposition in CO atmosphere are filtered on low adhesive force filters at the outlet. From there, the thin film can be transferred effectively to any substrate, e.g. flexible and transparent separator materials for EDLCs. SWCNTs produced with the aerosol method fulfil all the requirements for low-cost, flexible and transparent electronics that can be used in EDLCs [21–23].
In this work, dry deposited thin films of aerosol CVD synthesized SWCNTs are used as the electrodes in EDLCs. Remarkably high specific capacitance and stability are reached with the SWCNT electrodes in test cells and they are used to construct a transparent and flexible EDLC prototype.
2. Experiment
SWCNTs were synthesized by an aerosol (floating catalyst) CVD method based on ferrocene vapor decomposition in a CO atmosphere described elsewhere [24, 25]. Briefly, the catalyst precursor was vaporized by passing ambient temperature CO through a cartridge filled with ferrocene powder. The flow containing ferrocene vapor was then introduced into the high-temperature zone. In order to obtain stable growth of SWCNTs, a controlled amount of carbon dioxide was added together with the carbon source [26]. SWCNTs were collected downstream of the reactor by filtering the flow through nitrocellulose or silver membrane filters. It is worth noting that the SWCNTs produced by this method are of a high quality, which was confirmed with transmission electron microscope (TEM) examinations and optical investigations. Thus, they are immediately suitable for many applications without any additional treatment. Nevertheless, each SWCNT contains a catalyst particle encapsulated inside the tube and covered by graphitic carbon and cannot be washed out by HCl without opening the caps of the SWCNTs. The SWCNT film on different substrates was formed by a dry press transfer technique [19]. The film mass was weighed with a Mettler Toledo XP2 microbalance.
Structural and morphological characterizations of SWCNTs were probed with TEM (JEOL JEM-2200FS operated at 80 kV) and scanning electron microscopy (SEM, Zeiss sigma VP). Raman spectra were collected with a LabRAM HR ultraviolet–near-infrared (UV-NIR) instrument using back-scattered light from a 633 nm laser focused through a 50× objective lens. Absorbance measurements were carried out with a Perkin Elmer Lambda 950 UV–vis-NIR spectrophotometer.
The EDLCs were fabricated from SWCNT thin films as follows: a circular piece of Celgard 2300 (thickness 25 μm, diameter 1.5 cm) separator was cut and 1 × 1 cm piece of the thin film was transferred by simply pressing the separator against the film tightly. Thin films were transferred on both sides of the separator one after another. The SWCNT/separator was then dipped into 20 wt% HNO3 for 15 min to oxidize the SWCNTs and increase the hydrophilicity of the thin film [27]. Finally, the SWCNT/separator was washed with deionized water and placed in 1 M H2SO4 electrolyte overnight to saturate.
The electrochemical measurements were made in a Hohsen Corp test cell and two drops of electrolyte were placed in the cell along with the soaked SWCNT/separator to ensure proper wetting. The assembled EDLC was left to stabilize for 1 h before the start of the measurements and it was subsequently cycled between 0 and 0.6 V 25 times before any presented measurements were made. Cyclic voltammograms were recorded at scan speeds between 10 and 100 mV s−1. Constant current charging and discharging was performed at 0.53 A g−1 from 0 to 0.6 V and backward. The stability of the EDLC was studied by comparing the cyclic voltammogram recorded before and after cycling the potential between 0 and 0.6 V at 50 mV s−1 for 10 000 cycles. The EDLC was controlled and data recorded by an Autolab PGSTAT100 potentiostat (Metrohm).
A flexible and transparent EDLC prototype from SWCNT films was assembled as follows: an SWCNT film on 3 × 3 cm polyethylene terephthalate (PET) substrate was used as an electrode. A gel electrolyte was prepared by mixing a 5 wt% polyvinyl alcohol (PVA) solution (1 g in 10 ml deionized water) and concentrated H3PO4 (2 g of 85% H3PO4) for 1 h. The viscous PVA/H3PO4 solution was then applied on the SWCNT film with a brush to introduce a thin layer of PVA/H3PO4 on the SWCNT film surface. Two of these SWCNT films were then assembled face to face. Excess water in the PVA/H3PO4 gel on the SWCNT surface was eliminated by leaving the supercapacitor in a fume hood overnight. After water had been evaporated, the PVA/H3PO4 was solidified and bound the electrodes together.
3. Results and discussion
3.1. Properties of SWCNTs and their thin films
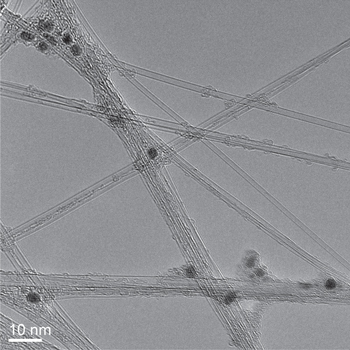
In figure 1, TEM images of SWCNTs are presented. Iron deposits from ferrocene used in the synthesis are visible and the iron content of the SWCNTs is 27 mol% from EDX. The SWCNTs have high specific surface area (950 m2 g−1) making them suitable for EDLC applications.
Figure 1. A TEM image of the SWCNTs.
Download figure:
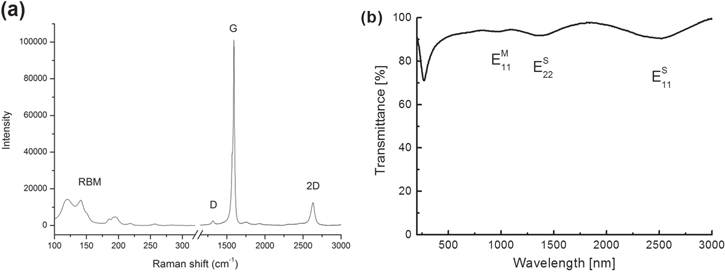
Standard image High-resolution imageIn figure 2(a) the Raman spectrum of the SWCNTs is presented. The D-band around 1350 cm−1 corresponds to defective structures while the G-band around 1580 cm−1 is indicative of graphitic structures [28]. The low intensity ratio between D and G bands (ID/IG = 0.029) shows that structure of the SWCNT is highly ordered and of good quality.
Figure 2. Properties of the SWCNT films. (a) Raman spectrum of the SWCNT film with 633 nm excitation wavelength laser. (b) Optical transmittance (92% at 550 nm) of the SWCNT film with mass density 3.10 μg cm−2.
Download figure:
Standard image High-resolution imageThe optical transmittance of the SWCNT film (figure 2(b)) is extremely high (77%–99%) in the visible light and NIR regions (250–2250 nm). Hereinafter, we refer to the transmittance measured in the middle of the visible range at 550 nm, which is 92% for this film. In the transmission spectrum electronic transitions in semiconducting (S11 and S22) and metallic (M11) SWCNTs, so called Van Hove singularities can be clearly observed. The mean diameter of SWCNTs estimated from the spectrum is about 2.2 nm [29], which corresponds to the TEM image. The roughness of the film is 30 nm [30] and the mass density of the film was determined to be 3.10 μg cm−2. The mass density of the other films (table 2) was estimated from their transparency:
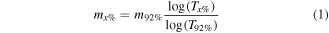
where mx% is the film mass (μg cm−2) with transparency Tx%.
Table 2. The properties of different SWCNT films and the specific capacitances of the EDLCs made from them.
| Transparency @ 550 nm (%) | Film mass μg cm−2 | Csp (total)a F g−1 | Csp (carbon)b F g−1 | Csp μF cm−2 |
|---|---|---|---|---|
| 92 | 3.1 | 178 | 482 | 552 |
| 85 | 6.0 | 173 | 467 | 1045 |
| 65 | 16.0 | 45 | 121 | 720 |
| 55 | 22.2 | 20 | 54 | 444 |
aSpecific capacitance of the SWCNT based on the total mass including iron residues. bSpecific capacitance of the SWCNT based on the carbon mass.
3.2. Electrochemical performance of the EDLC based on the SWCNT thin films
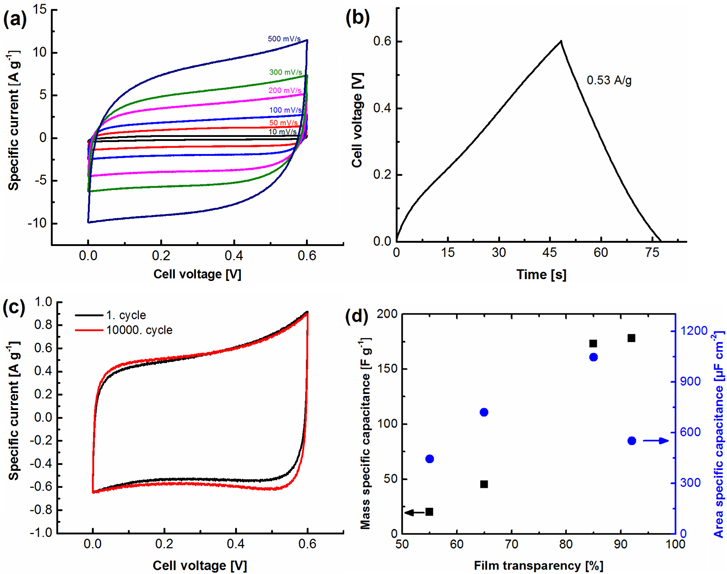
The electrochemical performance of the EDLCs made from the SWCNT thin films with 92% transparency was studied in a two-electrode cell (figures 3(a)–(d)). The cyclic voltammograms (figure 3(a)) show a rectangular shape that is ideal for EDLCs. The maximum potential (Vmax) is relatively low, 0.6 V, due to Faradaic processes starting at higher potentials. This is likely due to the Fe catalyst (27 mol%) remaining in the SWCNT film which can catalyze H2 evolution from water [31]. The specific capacitance of one SWCNT thin film electrode was determined from constant current charging (figure 3(b)) at 0.53 A g−1 between 0.5 Vmax and Vmax [32] from the formulas:
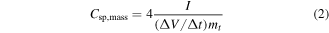

where I is absolute current, ΔV the change in voltage, Δt the change in time, mt is the total mass of two electrodes and Ageom is the geometric area of the supercapacitor (1 cm2). The multiplying factors 4 and 2 are derived from normalizing the specific capacitance for one electrode.
Figure 3. Electrochemical characterization of the SWCNT thin films as supercapacitors in 1 M H2SO4. (a) Cyclic voltammograms at various scan speeds (10, 50, 100, 200, 300 and 500 mV s−1). (b) Constant current charging and discharging at 0.53 A g−1. (c) Cyclic voltammogram of the supercapacitor before and after 10 000 cycles between 0 and 0.6 V. (d) The effect of the SWCNT film thickness on the mass and area specific capacitance of the EDLC.
Download figure:
Standard image High-resolution imageThis yielded specific capacitances of 178 F g−1 or 552 μF cm−2 for a single SWCNT electrode. When the specific capacitance is calculated only relative to the carbon mass of the SWCNT, the mass specific capacitance is naturally even higher (482 F g−1, table 2). The specific capacitance per electrode mass is extremely high and better than in other recent transparent and flexible EDLCs based on carbon nanomaterials presented in table 1. Remarkably, considering the low amount of carbon material used in the electrodes (3.10 μg cm−2), the area specific capacitance is comparable or larger than other EDLCs presented in table 1. This is likely due to the high surface area and graphitization of the SWCNTs (figure 2(a)) [33]. In figure 3(c), the cyclic voltammograms of the EDLC before and after 10 000 cycles between 0 and 0.6 V are shown and surprisingly, a small improvement in current is seen. This is most likely due to better wetting of the SWCNT films over time and indicates excellent stability for the SWCNT electrodes compared to other carbon EDLCs [11, 14, 15]. In combination with the very high optical transparency of SWCNT films, these properties make our SWCNT films an excellent material for flexible and transparent EDLC electrodes.
It is known that very thin films show high capacitances due to facile electrolyte transport [34–37]. Thus, when the film thickness is increased, the specific capacitance decreases to 20 F g−1 at 55% transparency corresponding to 22.2 μg cm−2 (figure 3(d)) showing the effect of longer electrolyte diffusion and electron transport through the layer. However, the specific capacitance normalized with the geometric area goes through a maximum at 85% transparency due to the mass specific capacitance decreasing while the mass of the electrode is increasing, indicating that by sacrificing a small level of transparency, the area specific capacitance can be doubled for SWCNTs used in applications demanding more energy. This enhancement is most likely caused by a smaller coverage of SWCNTs at high transparency. Capacitance values of EDLCs containing different film thicknesses are presented in table 2. The values are derived from constant current charging curves.
3.3. Transparent and flexible prototype EDLC
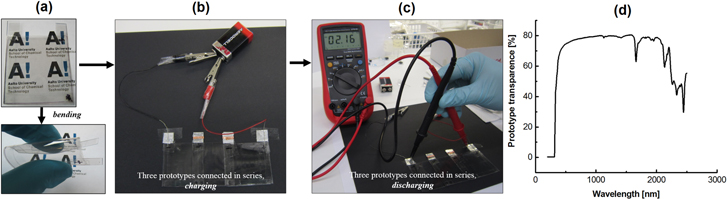
Finally, a flexible and transparent prototype was assembled from the SWCNT film with 92% transparency as electrodes, PET films as a casing and gel electrolyte. Its transparency and flexibility are demonstrated in figure 4(a). When three units were connected in series, total voltage of roughly 2.1 V could be achieved after charging for 1 min (figures 4(b)–(c)). The total optical transparence spectrum of one prototype cell is presented in figure 4(d) and its value in the visible and NIR region is very high 75%. This prototype proves the feasibility of using the SWCNT films in flexible and transparent EDLCs. A more extensive study of the properties of these films under repeated bending is underway and the results will presented in a future paper.
Figure 4. (a) Photos of a transparent and flexible EDLC prototype based on the thin SWCNT films. (b) Charging and (c) discharging of three EDLC prototypes connected in series. (d) The optical transparency of the single prototype cell in visible and NIR light.
Download figure:
Standard image High-resolution image4. Conclusions
We have used a facile, one-step aerosol synthesis method to fabricate thin SWCNT films and used a simple dry press transfer technique to apply them to different substrates. The transparency of the films is 92% at 550 nm and they are highly transparent in visible light and NIR wavelengths. The versatile transfer method allows easy combination of the SWCNT and substrate materials for transparent and flexible EDLCs. The films show extremely high mass specific capacitance of 178 F g−1 (482 F g−1 calculated per mass of carbon) and area specific capacitance of 552 μF cm−2 compared to other carbon based flexible and transparent EDLCs presented in the literature (table 1). The films are stable and their capacitance does not degrade during 10 000 loading cycles. Finally, a transparent, flexible and stackable EDLC prototype was fabricated from the SWCNT films. All in all, the notable conductivity, transparency, capacitance and stability of these easily transferable SWCNT films hold great promise in transparent and flexible electronics applications.
Acknowledgments
PK and TK thank the Jane and Aatos Erkko Foundation for funding of this research. NDL and LHS thank MIDE at Aalto University for funding. AGN and AT acknowledge the support by the Ministry of Education and Science of the Russian Federation through project RFMEFI 58114X0006. This work made use of the facilities of the Nanomicroscopy Center at Aalto University (Aalto-NMC).