Abstract
The evolution of pulsed laser deposition had been an exciting process of invention and discovery, with the development of high Tc superconducting films as the main driver. It has become the method of choice in research and development for rapid prototyping of multicomponent inorganic materials for preparing a variety of thin films, heterostructures and atomically sharp interfaces, and has become an indispensable tool for advancing oxide electronics. In this paper I will give a personal account of the invention and development of this process at Bellcore/Rutgers, the opportunity, challenges and mostly the extraordinary excitement that was generated, typical of any disruptive technology.
Export citation and abstract BibTeX RIS
I would like to take this opportunity, on the 25th anniversary of the advent of pulsed laser deposition (PLD), to recount my personal experience in the early days. One of the questions that emerges from these recollections is whether PLD as a thin-film deposition method for multicomponent materials was a discovery or an invention
We move back to 1986, the year of 'Supers'—the great supernova explosion, super Wall Street crash and high Tc superconductor (HTS) breakthrough. This came barely three years after the split up of Bell Labs creating a new Laboratory for the local telephone operating companies which came to be known as Bellcore and many of the more adventurous left Bell Labs for Bellcore. By the end of 1986 we had formed a robust group involved with HTS spearheaded by John Rowell, one of the early pioneers of superconductivity (he demonstrated the Josephson effect for the first time). My own group involved mainly with surface analysis and modification, was trying every way to gain a foothold in the field. It was a remarkable time for condensed matter physicists and the first major meeting on HTS at the New York Hilton Hotel was a standing-room only event. By the time the crowd dispersed in the early hours of the morning, many of us had made up our mind to become part of the extraordinary adventure opening before our eyes.
The people in my Bellcore team were supporting the main HTS thin films team with Rutherford backscattering (RBS), Auger and other measurements of film composition, and we realized that film deposition techniques such as sputtering and e-beam evaporation were having difficulty reproducing the composition of the multicomponent oxides. Part of my own research team at that time was based at Rutgers University (I was simultaneously a Founding Director of the Rutgers Laboratory for Surface Modification and Manager of the Surface Group at Bellcore), and we were heavily involved in laser surface interaction and at that time were looking at transient surface phenomena such as polymer ablation by short laser pulses and the relative importance of thermal versus photochemical effects. The 1987 spring MRS meeting which followed the APS was equally exciting as the interest in the field was exploding and prognostications of how our lives would be dramatically changed by the technology was being broadcast at regular intervals on the television. It was while I was at the MRS Meeting (Anaheim, CA) that the idea of using a pulsed laser to ablate a YBa2Cu3O7−x (YBCO) target occurred to me. I heard a talk by a Sandia scientist who was covering the surface of an object with ZrO2 using a pulsed Nd–Yag laser. So over lunch in Anaheim with my whole Bellcore team I broached the idea of using a pulsed UV laser to evaporate YBCO with the hope that maybe under a non-equilibrium evaporation condition we might able to preserve the stoichiometry of the YBCO. Virtually everyone on the table rejected the idea as an effort in futility. At Bell, we were always hypercritical about others' ideas, especially around the lunch table. But we were also thick-skinned. Rejection of ideas by close colleagues was often the fuel that led to new discoveries and inventions.
My Post doc Dirk Dijkkamp and graduate student Xindi Wu were then working in the Lab at Rutgers, and I suggested on the phone an experiment where they would irradiate a target of YBCO and with Excimer laser pulses with energy densities exceeding 1 J cm−2 to evaporate some of the material on to a carbon foil and run a Rutherford back scattering (RBS) spectrum of the foil. These numbers were extrapolated from the work we had done in the previous decade on pulsed laser annealing, another field where we were driven at maniacal levels of activity. For some reason I was extremely optimistic based purely on intuition rather than any scientific backing. So after the teleconference with my team at Rutgers I focused on the MRS meeting and when I got back to my Lab Saturday morning it was a rude disappointment to see no progress on the experiment. They had not managed to get a YBCO target (for heaven's sake even high schools were making these pellets in those days!). We were spurred into action immediately and people scurried across the campus looking for a ready-made YBCO target (which we located in Martha Greenblatt's Laboratory) and the first PLD experiment was performed in air. The laser-produced plume from the target was directed to a carbon foil and the resultant RBS spectrum is shown in figure 1.
Figure 1. Rutherford backscattering spectrum of a film deposited from a YBa2Cu3O7−x target on a carbon foil in air. A 30 ns, 248 nm laser pulse focused to an energy density of 1.5 J cm−2 was used [1].
Download figure:
Standard image High-resolution imageThe impact of these data was instantaneous. I called an emergency meeting of all my team members. We all realized the significance of these data and I realized right then that our lives were indeed changed for the better thanks to this technique. We had to put together an emergency auxiliary team for measurement of film composition, transport measurements and focused on making this a reproducible process and not a onetime occurrence.
Within a week we had made our first HTS film on sapphire which was post-annealed and showed a Tc of 75 K or so which was indeed inspiring to all of us. A paper we submitted to Applied Physics Letters was promptly rejected by a sceptical referee who claimed that this was most likely a lucky occurrence and unlikely to ever become a viable process for making these complex oxide films. We provided substantial evidence to the editor as to the number of different material systems where we were able to reproduce the composition and the paper was accepted for publication [1]. The PLD process became the flag bearer for Bellcore's efforts in HTS thin films and with the use of SrTiO3 substrates we were able to reach Tcs of 90 K rather quickly. Most of our announcements were met with a great deal of scepticism by the established superconductivity community, and to make matters worse there was a one month period when we were unable to produce any superconducting films whatsoever. The films, instead of being shiny black, were very non-uniform and would deteriorate rapidly in ambient conditions. It was clear that our control of the film composition was unpredictable.
We had existence proof that under suitable conditions we could reproduce the composition of the target faithfully but what were the critical parameters? At this stage we scoured the publications to see if our process had ever been attempted by someone else before and whether a recipe for this process existed. Ever since the invention of the ruby laser by Theodore Maiman at Hughes Research Laboratories in 1960, lasers were recognized as a source of energy for evaporating a variety of surfaces. The Department of Defence researchers found, much to their chagrin, that evaporating metals with lasers was a self-limiting process, as the plasma formed at the surface ended up soaking up most of the laser energy, thus limiting their potential for weapons (thank goodness!). There was substantial literature on using lasers to evaporate materials of all kinds, but no concrete evidence that pulsed laser evaporation would faithfully preserve the composition of a multicomponent material. At Rockwell, Halek Sankur and Jeff Cheung had a significant program on laser based evaporation and even though they had worked on numerous material systems there were no publications on preservation of composition of a multicomponent film using a pulsed laser [2]. At the time of HTS, their work was mainly concentrated on using cw and pulsed lasers to evaporate the films, and so the use of the lasers was primarily as a heat source and the benefits were not being fully exploited. While it was clear that pulsed lasers could be useful for evaporating a variety of materials, known from the time of the first ruby pulsed laser, a recipe for preservation of the composition of a multicomponent did not exist. We had to understand the process in a systematic way as quickly as possible—the race was on! It was back to the drawing board for us.
So we designed an experiment in which we created a point source of laser-produced plume and studied the angular distribution of the deposited film's thickness and composition using RBS. Another parameter that was varied was the laser pulse energy density. This result, shown in figure 2, clearly indicated the presence of two distinct components in the PLD plume, one with a highly forward-directed component Cosαθ, where α > 11 and a broad cos θ component [3]. We concluded by looking at the energy density dependence that the highly forward-directed component is the laser-produced plasma plume and the weaker angular component is the evaporative component arising from the residual heat in the target during and after the laser pulse.
Figure 2. At 1.5 J cm−2 the angular dependence of the deposited YBCO film (a) thickness and (b) composition measured via RBS [3].
Download figure:
Standard image High-resolution imageOn examining the composition as a function of angle it was very clear that the material deposited within an angle of about 20° with respect to the surface normal was stoichiometric and this component increased with the laser energy density (figure 3). Also, below a threshold energy density the composition was non-stoichiometric. This immediately told us that the highly forward-directed component (non-equilibrium component) is the desired stoichiometric one and the weaker angular dependent component (thermal component) was the undesired, non-stoichiometric one. This was the first real recipe for making PLD a reproducible process. One had to have a minimum energy density for a given material above which a stoichiometric deposition was possible, and the material had to be collected in a forward-directed angular cone not exceeding about 20° with respect to the surface normal. These ideas were patented by Xindi Wu and me [4]. By increasing the forward-directed component one gets more stoichiometric films which implied that the higher the energy density, the more stoichiometric the film composition will be. However, at very high energy densities the surfaces of the PLD targets tended to degrade and the particle density in the films was found to increase. Hence, a compromise in energy density was necessary.
Figure 3. Stoichiometry and thickness as a function of energy density: (a) and (b) 1.1 J cm−2, (c) and (d) 0.9 J cm−2, and (e) and (f) 0.5 J cm−2 [3].
Download figure:
Standard image High-resolution imageA reasonable picture of why PLD works started forming. An experiment involving a mass spectrometer revealed a few interesting facts, the material leaving the target consisted of a variety of species, dominantly mono and diatomic species and non-vanishing amounts of large molecular species such as BaCuOx, YCuOx and so on [5]. There were molecular species with kinetic energies approaching 1 keV and this of course was quite laser energy density dependent. In a background gas the velocity of the high energy species were substantially attenuated. So it was clear that the composition preservation depended on two extremely important factors,
- 1.The laser pulses pretty much removed a stoichiometric amount of material cumulatively over a number of laser pulses (not necessarily in a single pulse) and while all the material may not have been deposited with a single pulse, when averaged over a number of pulses the stoichiometry is preserved. This is a consequence of a non-equilibrium thermal evaporation process where the target temperature behind the plasma plume is not adequate to cause significant mass movement in the target either by segregation or by evaporation. As a result, any element that did not evaporate in the previous pulse will be removed in the subsequent pulse. It is pretty clear that this requires no mass movement in the target during and after the laser pulse. If there is substantial material flow from within to the surface or vice versa the composition will not be preserved. When dealing with a volatile constituent such as the alkali atoms for example, during the cooling phase of the target there is substantial loss of the alkali atoms from the surface, leading to a deficiency of these atoms in the film.
- 2.The second and most important question had to do with how the mass discrimination in the trajectory of the species is eliminated, which was essential for the stoichiometry conservation. The answer was in the fact that the vigorous collisions in the laser plume were necessary to remove the mass discrimination. The collision in the laser-produced plume tended to convert lateral momentum exchange into forward-directed momentum and further ensured that all the species had a similar average trajectory leading to composition preservation. The computations of Roger Kelley at IBM were some of the first to lend credence to these ideas [6].
There are numerous corollaries to the second factor though this sounds rather innocuous.
A top hat laser beam profile typical of excimer lasers is ideal for PLD as opposed to a TEM00 Gaussian pulse, as the lower energy tails in the Gaussian profile will contribute non-stoichiometric components.
This is the reason why for a given laser energy density there is an optimum target to sample distance beyond which the composition of a multi component target is not preserved—this happens when the plume density falls below an optimum density to preserve the required collisions.
This also explains the inverse relationship between the shape of the plume and the shape of the laser spot. The longer laser dimension produces a more forward-directed plume while the smaller dimension produces a more divergent plume, a resultant of density gradient in the plume.
This also explains why femtosecond lasers are a bad bet for PLD of multicomponent films. The longest plume possible with an FS laser will be of the order of a centimetre or less, since the amount of energy per pulse is much too small and so the sample-to-target distance will have to be very small.
For commercial production of PLD films, if one wants a large working distance, the laser spot will have to be bigger to provide a dense plasma plume over longer distances and thus, higher energy laser pulses would be required.
The laser rastering over the target becomes crucial as any changes in the surface morphology will direct the plume away from the substrate as the plume is always perpendicular to the target surface, and if the target is not uniformly eroded the plume will no longer be at the original position on the substrate, leading to off-stoichiometric films. Thus, the beam scanning requires scanning of a uniform top hat beam such that all parts of the target are uniformly eroded. A well-designed beam scanning system will result in a very smooth target with usage rather than a typical rough target surface. An idea that I credit to Doug Lowndes of ORNL and Ross Muenchausen at Los Alamos is the requirement to hit the same spot on the target by a laser pulse from two opposing directions whereby the feature developed by shadowing effect of a small protrubence will be etched away by a beam from the opposing direction. Otherwise one would generate a surface profile analogous to a missile silo [7]!
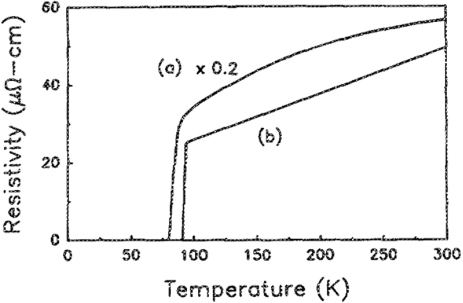
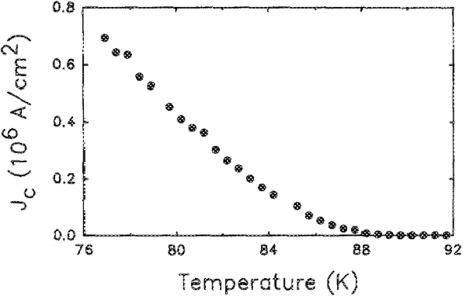
We realized relatively quickly that the substrate is crucial for producing high-quality YBCO films and as soon as we tried SrTiO3 the Tcs approached 90 K and the critical current densities those of single crystals (figures 4 and 5). Further, depositing the YBCO films under high partial pressures of oxygen (∼100 mT) followed by a soak of the film during cooling at a fraction of an atmosphere resulted in in situ grown superconducting films of high quality [8]. Our own work accelerated in pace with healthy competition from IBM Yorktown Height's A Gupta's team [9], whose outstanding work in the fabrication of a variety of HTS films including in situ YBCO, BSSCO and the electron doped NCCO films for the first time greatly inspired us, validated our work and kept us focused. An equally important contributor to this field was Koinuma and Kawasaki's group in Tokyo Institute of technology where I could see the emergence of an oxide power house.
Figure 4. ρ versus T curve for an as-deposited 0.2 mm Y1Ba2Cu3O7−δ film on (a) Al2O3 at 580 C and (b) (1 0 0) SrTiO3 at 650 C. The zero resistance is seen at 78 K and 88.6 K, respectively [8].
Download figure:
Standard image High-resolution imageFigure 5. Critical current density as a function of temperature for an as-deposited YBa2Cu3O7−δ film on (1 0 0) SrTiO3 [8].
Download figure:
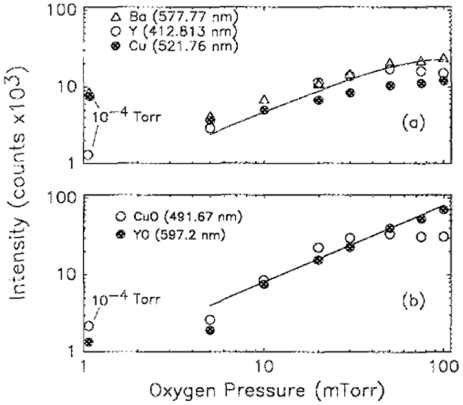
Standard image High-resolution imageThe mechanism of the in situ deposition of YBCO films under high oxygen pressures of 100 mT became obvious once we looked at the optical spectra of the plume whose colour had changed from blue to pink. This was primarily the emission from molecular YO dominating over the emission from the elemental lines which tended to be at higher energies (figures 6 and 7) [9]. So during in situ deposition, the species in the plume were reacting with the ambient atmosphere, thereby increasing the chances for forming an as-deposited epitaxial film with the right oxygen stoichiometry. However, the most experienced researchers in my lab would just look at the plume and with their calibrated eyesight tell whether the conditions were optimum or not for good YBCO films!
Figure 6. Optical spectra of laser (248 nm, 30 ns) produced plume from a YBa2Cu3O7−δ target at an oxygen pressure of (a) <10−4 Torr, (b) 20 mT and (c) 100 mT. The spectra were taken at a laser energy density of 1.5 J cm−2. The background has been subtracted from the spectra [10].
Download figure:
Standard image High-resolution imageFigure 7. Oxygen pressure dependence of (a) the elemental lines and (b) the oxide lines. The solid line in (a) is an empirical fit of P → kP2, where P is the oxygen pressure and k ∼ 5.5 × 10−3 mT−1. The solid line in (b) is a linear fit of P [10].
Download figure:
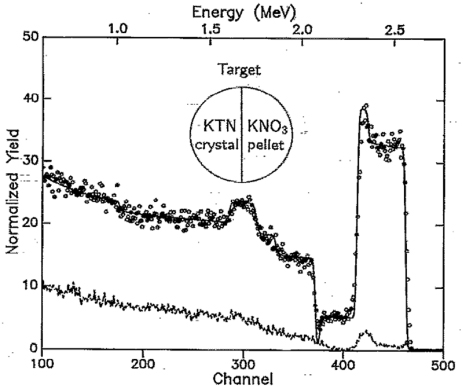
Standard image High-resolution imageGoing back to the issue of non-equilibrium evaporation it is now easy to understand why when the target contains a highly volatile element, the composition preservation becomes a problem. We faced this problem in collaboration with Heinrich Hertz Institute (Berlin) who had a great interest in electro-optic films for high-density TV applications. One of the star materials for this is KTaNbO3 with a huge electro-optic coefficient. The K in the target was a serious problem in terms of film stoichiometry. At the residual temperatures of the target after the laser pulse, potassium will continue to evaporate (equilibrium thermal evaporation) leading to a depletion of K in the target surface, leading to K depletion in the film as well. The problem was solved by making a segmented target where one half was KNO3 and as the laser pulses struck the KNO3 part of the target an excess of potassium was provided in the laser plume which then compensated the loss of K [10]. The assimilation of K in the film was limited then by the film stoichiometry and any excess K would not stick to the film, leading to perfect stoichiometry. These films were highly crystalline and were of the right composition and exhibited large electro-optic effect (figure 8).
Figure 8. RBS spectrum (He2+) of a random-oriented epitaxial KTN film on (1 1 0) SrTiO3 (circles), RBS channelling spectrum of the same sample (dashed line) and RBS simulation of KTa0.67Nb0.33O3/SrTiO3 (solid line) [11].
Download figure:
Standard image High-resolution imageEven by this time scepticism about PLD process did not abate, and our attitude was to let the data speak for themselves. John Clarke at UC Berkeley was sent some of our PLD films which he compared with films deposited by off-axis sputtering from Stanford and films made locally at Berkeley. Amazingly, the flux noise in the PLD films turned out to be orders of magnitude smaller than in other films (figure 9), and this result clearly started the turnaround for PLD process and very soon the credibility for the process was established and there was a sudden increase in the number of research laboratories working on PLD [12].
Figure 9. Flux noise versus temperature for YBCO films deposited by PLD [12].
Download figure:
Standard image High-resolution imageOne of the most exciting and enduring collaborations was with Ramesh who joined Bellcore as a TEM expert and after working with us, branched out into using PLD for ferroelectric films and heterostructures where his work had a significant impact. We continued our interaction in oxide electronics beyond Bellcore for a decade or more at the University of Maryland.
By May 1989, I had started a company, Neocera Inc., in order to create oxide electronics based products. PLD systems became one of our first products. Today PLD has evolved into a major deposition process and in conjunction with high pressure RHEED, demonstrated for the first time by the Twente group [13], is able to produce atomically sharp interfaces of dissimilar materials. The invention of the PLD process has been a great enabler for the field of oxide electronics which accelerated over the last 25 years. Industrial scale PLD systems have been made with the ability to deposit films over 8 inch wafers with thickness uniformities approaching ±5% or better and particle (100 nm or larger) densities below 10 per square inch [14]. High Tc production processes use PLD at various stages of the process but still I believe PLD is a process looking for a niche application, most likely the deposition of a high-value multi component film. Today, researchers in the field of a variety of multicomponent materials such as superconductors, manganites, ferroelectrics, multiferroics and so on, predominantly use PLD as the deposition tool of choice primarily because of the rapid prototyping capability of this technique. If you want to synthesize a stoichiometric, epitaxial film of a new material, the chances are that PLD may be the fastest way to get there.
Returning to the issue of whether PLD was an invention or a discovery, I would define an invention as an advance that arises as a result of deliberate rational analysis, and a discovery as one that comes about by chance. In that case, it seems clear to me that PLD should be considered as an invention. After we discovered the dual component in the PLD plume, Wu and I filed a patent in 1989 where a primary claim was that material is collected within an angle of 20°–25° in a PLD process. We also defined specific energy density ranges for specific materials and laser wavelengths. However, the strength of the claims was considerably weakened by the examiner's insistence that we specify the insertion of an angle-confining aperture in the laser plume rather than accept the original claim where we simply collected the film material within a forward angle of 20°–25°. If the original claim had been allowed then this would have been a crucial patent in the field of pulsed laser deposition. We know that the insertion of an aperture or anything else in the PLD plume is not recommended, as the plume can sputter the material into the deposited film. As a matter of fact, most metallic surfaces are kept as far away from the plume as possible to prevent this sputter induced contamination. This is also the reason why a very small PLD chamber will not be effective in preserving the film composition. Small chambers may be best suited for fs laser based system where the working distance has to be very small owing to the small plume. Nevertheless, the PLD we see today qualifies as an invention because the conditions under which the composition is preserved were described for the first time.
The evolution of PLD has been an exciting process of invention and discovery, with the development of high Tc superconductors as one of its biggest collateral benefits. It has become the method of choice for preparing all sorts of oxide thin films and heterostructures, and an indispensable tool for advancing oxide electronics. The program with its beginning in Bellcore/Rutgers involved collaborations with numerous Universities, corporations and National Labs and what we have achieved today is a testament to this extraordinary pioneering team effort.
Acknowledgments
I would specifically like to thank, Xindi Wu, Dirk Dijkkamp, Arun Inam, Alp Findikoglu, Satish Ogale, M S Hegde, R Ramesh, B Datta, J M Rowell (members of the original team) and E W Chase, L Nazar, S A Schwartz, S W Chan, B Wilkens, J M Tarascon, S Gregory, P Lindenfeld, the late WS Mclean, Martha Greenblatt, Nadjeh Jisrawi, C J Palmstrom and N G Stoffel, all from the Bellcore/Rutgers team.
I must mention the pioneering work of Gad Koren, Arunava Gupta at IBM, Hideomei Koinuma and Masashi Kawasaki at TIT Japan who kept the competitive spirit going in this field, propelling us go higher.
In addition, I would like to thank A Pique, S Green, S Harshavardhan, J Kim and M Strikovski, original members of Neocera, who have literally taken the technology to new heights with sales in 26 countries around the world. I consider myself extraordinarily fortunate to have played a pioneering role in the development of this process as it enabled me to tackle some of the most exciting problems in condensed matter physics but also brought me into collaborative work with some of the most creative and enterprising minds.