ABSTRACT
Large isotopic anomalies are found in meteoritic insoluble organic materials (IOMs) and, for nitrogen, show 15N-excesses up to  N ∼ 5000‰. These 15N-enrichments are commonly ascribed to presolar origins, but the attribution seems contradicted by available data on N-isotopes' cosmic distribution. We report here that 15N hotspots in several IOMs are reduced by hydrothermal treatment and their loss correlates with 15N values of ammonia released upon treatment. Because released ammonia's 15N-enrichments also relate with meteorites' mineralogy, i.e., asteroidal processes, and no current models offer plausible explanations for the finding, we account for our data with a novel scenario whereby 15N-enriched ammonia produced in the solar nebula is incorporated by carbonaceous materials and delivered to early Earth by comets and meteorites. The proposal also implies that abundant reduced nitrogen, a required element in origins of life theories, could reach our nascent planet and other planetary systems affecting their habitability.
N ∼ 5000‰. These 15N-enrichments are commonly ascribed to presolar origins, but the attribution seems contradicted by available data on N-isotopes' cosmic distribution. We report here that 15N hotspots in several IOMs are reduced by hydrothermal treatment and their loss correlates with 15N values of ammonia released upon treatment. Because released ammonia's 15N-enrichments also relate with meteorites' mineralogy, i.e., asteroidal processes, and no current models offer plausible explanations for the finding, we account for our data with a novel scenario whereby 15N-enriched ammonia produced in the solar nebula is incorporated by carbonaceous materials and delivered to early Earth by comets and meteorites. The proposal also implies that abundant reduced nitrogen, a required element in origins of life theories, could reach our nascent planet and other planetary systems affecting their habitability.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
Carbonaceous Chondrite (CC) meteorites are asteroidal fragments of near solar elemental abundances that avoided the geological reprocessing of planet formation. CC stones have the appearance of aggregate rocks and contain carbon as soluble and insoluble organic materials (IOMs), intermixed with silicates and other inorganic phases diverse enough in composition and 18O/16O fractionation to allow their classifications into various groups. Their studies have provided insights on primordial Solar System constituents as well as temperature and compositional parameters of early solar nebula events (Russell et al. 2006). In regard to the cosmic evolution of carbonaceous materials, CC meteorites' analyses demonstrated that abiotic organic materials preceded biochemistry in the early solar system and could be as diverse as kerogen-like complex macromolecules and simpler soluble compounds varying from polar species such as amino acids or polyols to non-polar hydrocarbons (e.g., Pizzarello & Shock 2010). Furthermore, the finding that CC compounds may display high D-enrichments (Pizzarello & Huang 2005) comparable to those observed in molecular clouds of the interstellar medium (ISM) indicates that HCNO-containing interstellar precursors could survive in asteroidal bodies. Given that most CC parent bodies are known to have been further processed during their early solar residence, with internal heating (∼0°C–125°C), consequent release of liquid water and associated effects on their minerals such as the formation of clays (Brearley 2006), it has been generally understood that the presolar precursors were further processed via aqueous reactions into the organic molecular species found upon meteorites' extraction.
This interstellar-parent body formation hypothesis (Cronin & Chang 1993) has one lacuna, however, and that is that the 15N anomalies of several CC compounds cannot be easily explained by interstellar processes since large 15N enrichments in general, and for the ammonia envisioned in some of these reactions (Lerner & Cooper 2005) in particular, have not been detected in the ISM in spite of their prediction. Some of the latest cosmic abundances measured for 14N/15N ratios were: 290 ± 40 for the local ISM (Adande & Ziurys 2012), ∼350–850 (Gerin et al. 2009) and 334 ± 50 (Lis et al. 2010) for ammonia specifically, and 424 ± 3 for protosolar environments, taken as the nitrogen composition of the solar wind measured during the Genesis mission (Marty et al. 2010); the ratio of the terrestrial atmosphere is +272. The corresponding values in the geochemical  N‰ notation used for meteorites (see Table 1) are, respectively: −84.7‰, −222 to −680‰, −186‰, and −374‰, where 0 is the terrestrial atmosphere.
N‰ notation used for meteorites (see Table 1) are, respectively: −84.7‰, −222 to −680‰, −186‰, and −374‰, where 0 is the terrestrial atmosphere.
Table 1. Average δ15N Values of Largest "Hotspots" in Individual Meteorites' IOM and HT IOM, HT Released Ammoniaa, and Bulk IOMb
| IOM | HT-IOM | |||||||
|---|---|---|---|---|---|---|---|---|
| Meteorite Name |
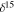 N‰ N‰ |
# | μm2 Area Mounts |
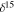 N‰ N‰ |
# | μm2 Area Mounts |
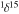 N‰ NH3 N‰ NH3 |
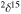 N‰ Bulk IOM N‰ Bulk IOM |
| Murchison (CM2) | 568 ± 143 | 5 | 5400 | 456 ± 146 | 1 | 3050 | 51.7 | −1 ± 7.5 |
| Ivuna (CI1) | 1323 ± 146 | 4 | 4125 | 548 ± 130 | 9 | 5831 | 66.4 | 31.9 |
| Bells (C2-ung) | 1412 ± 249 | 8 | 1900 | 1178 ± 120 | 12 | 700 | 455.5 | 415 ± 1.6 |
| Tagish Lake (C2-ung) | 1620 ± 198 | 7 | 4675 | 1436 ± 128 | 3 | 4050 | 140 | 130 ± 1.5 |
| GRA 95229 (CR2) | 1364 ± 118 | 13 | 5175 | 919 ± 241 | 3 | 3150 | 223 | 153 ± 6.7 |
| Allende (CV3) | nf | ⋯ | 2250 | nf | ⋯ | ⋯ | nf | −51 ± 0.6 |
| Sutter's Mill (C-ung) | nf | ⋯ | 2220 | nf | ⋯ | ⋯ | nf | nm |
Notes.  N‰ = [(15N/14Nsample/15N/14N
N‰ = [(15N/14Nsample/15N/14N  −1] × 103; 15N/14Nstandard is the ratio of terrestrial atmosphere (3.676·10
−1] × 103; 15N/14Nstandard is the ratio of terrestrial atmosphere (3.676·10
 N‰ = 0). #, indicates the number of hotspots identified in at least 0.03 mm2 of each sample; nf, not found; the acronyms within parentheses after the meteorites' name indicate: their class (C, for carbonaceous, plus the initial letter of the prototypical meteorite, e.g., M for Mighei; I for Ivuna etc.) and petrologic type (1–3, 1 = maximum water alteration). Sutter's Mill and Allende did not display hotspots or released ammonia upon HT treatment and were included as procedural blanks.
N‰ = 0). #, indicates the number of hotspots identified in at least 0.03 mm2 of each sample; nf, not found; the acronyms within parentheses after the meteorites' name indicate: their class (C, for carbonaceous, plus the initial letter of the prototypical meteorite, e.g., M for Mighei; I for Ivuna etc.) and petrologic type (1–3, 1 = maximum water alteration). Sutter's Mill and Allende did not display hotspots or released ammonia upon HT treatment and were included as procedural blanks.
Download table as: ASCIITypeset image
Only limited 15N isotopic fractionation by ion molecule reactions has been considered possible for interstellar clouds in some studies, based on nitrogen's slight fractional ionization (Terziera & Herbst 2000), while other models predicted even large enrichments, e.g., for nitrile-containing compounds and ammonia in CO depleted ISM regions (Rodgers & Charnley 2008a, 2008b) or based on spin states dependence between N2 and H2 in ion-molecules reactions (Wirström et al. 2012). So far, however, 15N-enrichments in the ISM have been measured only for HCN, and within broad ranges and diverse environments. Observations of H13CN and HC15N rotational transitions estimated 14N/15N ratios of 140–360 in protostellar cores (Hily-Blant et al. 2013), added measurement of C14N and C15N lead to 120–400 ratios across the galaxy (Adante & Ziurys 2012) and a search for extragalactic HC15N inferred ratios of 111 ± 17 (Chin et al. 1999).
By contrast, large 15N-enrichments have been detected consistently in small bodies of the Solar System: spectroscopically for HCN and CN in comets (Bockelée-Morvan et al. 2008; Manfroid et al. 2009), by Secondary Ion Mass Spectroscopy (SIMS) for micrometer sized interplanetary dust particles (IDPs) collected in the high atmosphere (Messenger 2000), and more frequently in meteorites, which, being amenable to direct laboratory analyses, allowed measurements of positive 15N/14N enhancements for several N-containing classes of compounds, amines (Pizzarello et al. 1994), amino acids (Engel & Macko 1997; Pizzarello & Holmes 2009; Elsila et al. 2012), ammonia (Pizzarello et al. 2011), and polar hydrocarbons (Pizzarello et al. 1994), with  values for these molecules reaching ∼250‰. Above all, it was the IOM of CC meteorites that revealed the highest
values for these molecules reaching ∼250‰. Above all, it was the IOM of CC meteorites that revealed the highest  isotopic fractionations, which are found in the form of localized submicron-sized areas showing 15N isotopic anomalies as high as
isotopic fractionations, which are found in the form of localized submicron-sized areas showing 15N isotopic anomalies as high as  N ∼ 5000‰ (Busemann et al. 2006; Briani et al. 2009) and called "hotspots," as for similar anomalies of other elements.
N ∼ 5000‰ (Busemann et al. 2006; Briani et al. 2009) and called "hotspots," as for similar anomalies of other elements.
The IOM represents the major portion of the carbon in CCs, up to ∼99% by weight, and is often compared to terrestrial kerogens for its macromolecular complexity, insolubility, and content of H, O, N, S, and P in addition to C. The IOM is highly heterogeneous and several of its chemical and isotopic features are still not fully understood, e.g., its precise chemical composition. It was long believed that polycyclic aromatic hydrocarbons (PAHs) were the primary IOM components (Allamandola et al. 1989), however, recent findings assigned the infrared features attributed to PAHs to combined aromatic and aliphatic components of no fixed arrangements (Kwok & Zhang 2011). Experimental studies have also proposed a new pathway for producing complex presolar aggregates (Schutte et al. 1993), and IOM formation in particular (Cody et al. 2011), via polymerization of formaldehyde, an abundant component of the ISM organic inventory and, presumably, the solar nebula.
It is plausible, in fact, that the IOM is a mixed aggregate of several phases and functional groups as indicated by pyrolytic and Nuclear Magnetic Resonance (NMR) studies (e.g., Sephton et al. 1998; Cody et al. 2002). We showed in previous work that the IOM, while considered resistant to chemical treatment, could release a large suite of solvent-soluble compounds by hydrothermal treatment (HT), i.e., conditions similar to those of terrestrial hydrothermal vents (300°C, 100 MPa) (Yabuta et al. 2007). More recent experiments revealed that several IOMs from meteorites belonging to diverse CC classes also hydrothermally release abundant ammonia (NH which represents in some instances the largest molecular component of the HT extracts, with 15N enrichments of +45‰ to +455‰. In these studies, the detection of ammonia by Gas Chromatography-Mass Spectrometry (GC-MS; Pizzarello & Williams 2012) and identification of its individual 15N enrichment by Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS; Pizzarello et al. 2011), i.e., by compound specific analytical techniques, excluded interferences by any possible terrestrial contaminants or other co-eluting water-soluble compounds (see below for possible exceptions in regards to CI meteorites in this study).
which represents in some instances the largest molecular component of the HT extracts, with 15N enrichments of +45‰ to +455‰. In these studies, the detection of ammonia by Gas Chromatography-Mass Spectrometry (GC-MS; Pizzarello & Williams 2012) and identification of its individual 15N enrichment by Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS; Pizzarello et al. 2011), i.e., by compound specific analytical techniques, excluded interferences by any possible terrestrial contaminants or other co-eluting water-soluble compounds (see below for possible exceptions in regards to CI meteorites in this study).
The findings were surprising in view of the insoluble composition of IOM and the harsh chemical processing involved in isolating the material from the minerals (Cronin et al. 1987), which would easily eliminate any surficial ammonia or ammonia-containing compounds. Even more unexpected, however, was discovering that the 15N isotopic enrichments of the IOM-released ammonia related closely with the classification of the meteorites (Pizzarello & Williams 2012), i.e., with the mineralogical make-up and alteration history of the stones. Because the mineralogical composition of CC meteorites is considered to be largely the result of pre- and post-accretional processes affecting solar aggregates, their precursors and/or asteroidal parent bodies (e.g., Clayton & Mayeda 1984), these data inferred that such Solar processes were also involved in IOM ammonia's formation/acquisition as well as of its isotopic enrichments.
Aiming to better understand these data, we have analyzed samples of IOM powders from the same meteorite types used in the Pizzarello & Williams (2012) study, both per se and after HT, and measured the extent of 15N fractionation in 15N-rich "hotspots" to evaluate their possible isotopic relation to IOM-released ammonia. Although the analytical comparison involved IOM domains of different scales, it was allowed by the blending effect of the demineralization process, which involved protracted acid extractions, repeat rinses, multiple solvents and sonications to yield just few mg g−1 of near to homogeneous IOM powders (see materials and methods). Owing to the heterogeneous distribution of the N-anomalous hotspots in meteoritic IOMs, as seen by several authors (e.g., Briani et al. 2009; Bose et al. 2014), we measured equivalent sizes and several different areas for all samples in this study (Table 1), except for Bells. Analyses of the "hotspots" were performed by nanoscale Secondary Ion Mass Spectrometry (NanoSIMS) on comparatively (Messenger 2000; Busemann et al. 2006) large areas to statistically evaluate the data. Since "hotspots," in spite of their name, have broad fractionation ranges up to terrestrial values, we chose for our study anomalies at or above 3σ from the terrestrial range.
2. METHODS
All meteorite powders were extracted sequentially with water, dichloromethane/methanol (9:1, v:v), carbonyl disulfide, and 6NHCl, with drying after each step, and then demineralized as previously described (Cronin et al. 1987). Briefly, powders were treated with 30 ml of 8N HF/3N HCl solution in water, in Teflon containers, at  °C for five days with stirring and then repeatedly rinsed with 6N HCl, 3NHCl, and water with intermittent sonications until reaching a stable weight upon drying. The materials were then further extracted in sequence with water and MeOH at 100°C for 2hrs. The meteorites used for the experiments and their weight % recoveries versus starting powders were: Orgueil: 8.0; Ivuna: 4.5; Murchison: 2.7; Bells: 3.0; Tagish Lake: 3.9; GRA 95229: 2.6; Allende: 0.3; Sutters' Mill: 1.8.
°C for five days with stirring and then repeatedly rinsed with 6N HCl, 3NHCl, and water with intermittent sonications until reaching a stable weight upon drying. The materials were then further extracted in sequence with water and MeOH at 100°C for 2hrs. The meteorites used for the experiments and their weight % recoveries versus starting powders were: Orgueil: 8.0; Ivuna: 4.5; Murchison: 2.7; Bells: 3.0; Tagish Lake: 3.9; GRA 95229: 2.6; Allende: 0.3; Sutters' Mill: 1.8.
2.1. Hydrothermal Experiments
The HT treatment was also previously described (Pizzarello et al. 2011) and was conducted with 10–15 mg of IOM powders, at 300°C and 100 MPa for six days, in pure gold tubes sealed under Ar gas. Molecular analyses of ammonia and other N-containing compounds in the extracts were conducted by Gas Chomatography-Mass Spectrometry, also as in the above reference.
2.2. NanoSIMS Analyses
The IOM and HT-treated IOM powders were ultrasonicated in methanol and small volumes of the material were pipetted onto ultra-clean gold foils adhered on 10 mm diameter stainless steel stubs. Isotopically anomalous "hotspots" are defined as micron-to submicron-sized regions in the ion image that exhibit 15N excesses, where each hotspot has  N‰ value ≥3σ deviation from terrestrial average ratio and shows anomaly in more than three layers for a given measurement. Analyzed areas covered >75% of the sample material. The
N‰ value ≥3σ deviation from terrestrial average ratio and shows anomaly in more than three layers for a given measurement. Analyzed areas covered >75% of the sample material. The  values were acquired with a NanoSIMS 50L using standard protocols (Bose et al. 2012). Succinctly, a 16 keV, <1 pA Cs+ primary ion beam with a beam diameter of <100 nm sputtered the sample surface, while acquiring mass filtered images of 12C−, 13C−, 12C
values were acquired with a NanoSIMS 50L using standard protocols (Bose et al. 2012). Succinctly, a 16 keV, <1 pA Cs+ primary ion beam with a beam diameter of <100 nm sputtered the sample surface, while acquiring mass filtered images of 12C−, 13C−, 12C , 12C14N−, 12C15N−, 28Si− plus secondary electrons in multicollection mode. A 50–100 pA Cs+ beam was used to pre-sputter for ∼5 minutes to achieve steady state sputtering. Each ion image is composed of a stack of 6–10 cycles. For each cycle, 10 × 10 μm2 or 15 × 15 μm2 regions of the sample mount decomposed into 256 × 256 pixels were measured for 10–15 ms/pixel dwell time. Cyanoacrylate "Krazy glue" was used for tuning and external normalization of the isotopic ratios.
, 12C14N−, 12C15N−, 28Si− plus secondary electrons in multicollection mode. A 50–100 pA Cs+ beam was used to pre-sputter for ∼5 minutes to achieve steady state sputtering. Each ion image is composed of a stack of 6–10 cycles. For each cycle, 10 × 10 μm2 or 15 × 15 μm2 regions of the sample mount decomposed into 256 × 256 pixels were measured for 10–15 ms/pixel dwell time. Cyanoacrylate "Krazy glue" was used for tuning and external normalization of the isotopic ratios.
2.3. Materials
Solvents were high purity and once distilled, HCl and HF solutions in water were twice distilled and water was triply distilled. Glassware and gold tubes were kept at 500°C overnight before use.
3. RESULTS AND DISCUSSION
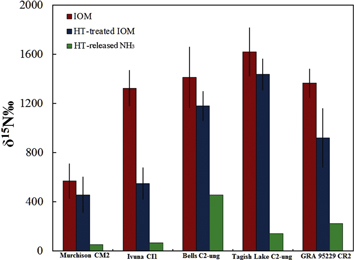
Data are listed in Table 1 and summarized in Figure 1; they present a quantitative overview of the 15N isotopic enrichments detected before and after HT treatment from equivalent areas of individual IOMs, with their standard deviations and in comparison to those of their IOM-released ammonia and bulk IOM15N (Alexander et al. 2007). Figure 2 gives a sample NanoSIMS image collected in the study.
Figure 1. Histogram comparing δ15N‰ values of meteoritic IOM hotspots, HT-IOM hotspots, and IOM-released NH3 for each meteorite. See methods for preparation and analyses of the samples. Allende and Sutter's Mill had no extractable ammonia or 15N-hotspots and data from them were used as secondary reference standards. Ammonia values from Pizzarello & Williams (2012).
Download figure:
Standard image High-resolution imageFigure 2. NanoSIMS ion images of hydrothermally treated Ivuna IOM. NanoSIMS 50L ion images show the distribution of: (a) 12C−, (b) 12C14N−, (c) 12N 15N− of the 15 × 15 μm2 area in the mount containing HT-IOM Ivuna. The CN images are used to find the hotspot locations that are characterized by 15N excesses. The maps (256 × 256 pixels) of HT-IOM spotted on ultra-clean Au foils are derived from an image stack of seven images, 20 ms dwell time/pixel. (d) Delta 15N image created by using the counts/s/pixel from the CN− images (see methods); this sample, of CN-/C- ratio of ∼0.4, reveals the 15N anomaly (encircled in red) and shows the largest 15N excess ( = 548 ± 130‰) in HT-IOM Ivuna against the rest of the image (in shades of blue) that has
= 548 ± 130‰) in HT-IOM Ivuna against the rest of the image (in shades of blue) that has  ∼ 0. The image's applied false color has a scale where red implies high 15N values and blue implies low 15N values. The other regions of interest in the delta image that exhibit
∼ 0. The image's applied false color has a scale where red implies high 15N values and blue implies low 15N values. The other regions of interest in the delta image that exhibit  ∼200‰–400‰ do not have enrichments within
∼200‰–400‰ do not have enrichments within  -errors compared to cyanoacrylate standard.
-errors compared to cyanoacrylate standard.
Download figure:
Standard image High-resolution imageAs shown, 15N anomalies are found to be ubiquitous in all IOMs analyzed, except for the Allende and Sutter's Mill meteorites, whose IOMs did not release ammonia upon HT (e.g., Pizzarello & Williams 2012; Pizzarello et al. 2013), for which no anomalies were found and whose data were taken as baseline reference. The 15N anomalies are reduced in number after HT to an extent that is compatible with their release of ammonia.
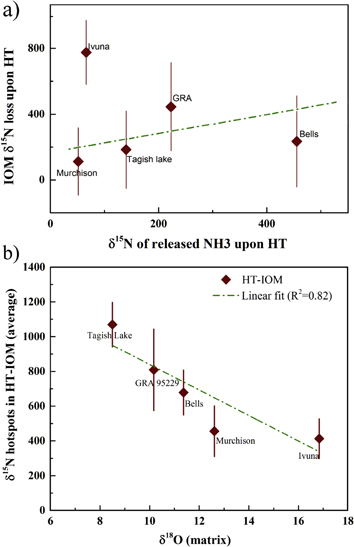
The correlation between IOMs  N‰ reduction upon HT and IOM-released ammonia is given in Figure 3(a), it shows a trend of the data that is consistent with our proposal with the exception of Ivuna's. The remaining variation from linearity, e.g., for GRA, can be reasonably attributed to the small IOM amounts involved in the analytical technique, the inherent difference in samples' areas between repeat analyses and, as referenced above, the known heterogeneity of the IOM.
N‰ reduction upon HT and IOM-released ammonia is given in Figure 3(a), it shows a trend of the data that is consistent with our proposal with the exception of Ivuna's. The remaining variation from linearity, e.g., for GRA, can be reasonably attributed to the small IOM amounts involved in the analytical technique, the inherent difference in samples' areas between repeat analyses and, as referenced above, the known heterogeneity of the IOM.
Figure 3. (a) Correlation between IOM's δ15N‰ reduction upon hydrothermal treatment and IOM-released ammonia. (b) Average δ15N values of measured hotspots in HT-IOM samples versus matrix δ18O ratios. Ratios were taken from published literature (Clayton & Mayeda 1999); the  of matrix GRA 95229 is not available and data for the CR2 Renazzo in the above reference were used instead.
of matrix GRA 95229 is not available and data for the CR2 Renazzo in the above reference were used instead.
Download figure:
Standard image High-resolution imageIvuna IOM data have instead clearly different values and, even if showing hotspots losses upon HT, gave larger  N-reductions compared to released ammonia than other meteorites. We measured IOM and HT IOM area (2500 μm2) of Orgueil, a meteorite of the same type as Ivuna, and detected an even larger 15N-reduction, 1075 (not listed because the smaller quantities available for these IOMs prevented repeat analyses). Both meteorites are classified as CI, a rare type of stones, of which only four falls are known and one specimen (Alais, fallen in 1806) is no longer available (Sears & Dodd 1988). CIs show in their predominant clay composition and absence of chondrules, a pervasive asteroidal alteration by water, and have been at times likened to comets (e.g., Lodders & Osborne 1999). The deviation from the trend displayed in 3a by both CIs suggests that their protracted parent body alteration might have affected bondings within their IOMs and led to a larger release of organic compounds besides ammonia upon HT.
N-reductions compared to released ammonia than other meteorites. We measured IOM and HT IOM area (2500 μm2) of Orgueil, a meteorite of the same type as Ivuna, and detected an even larger 15N-reduction, 1075 (not listed because the smaller quantities available for these IOMs prevented repeat analyses). Both meteorites are classified as CI, a rare type of stones, of which only four falls are known and one specimen (Alais, fallen in 1806) is no longer available (Sears & Dodd 1988). CIs show in their predominant clay composition and absence of chondrules, a pervasive asteroidal alteration by water, and have been at times likened to comets (e.g., Lodders & Osborne 1999). The deviation from the trend displayed in 3a by both CIs suggests that their protracted parent body alteration might have affected bondings within their IOMs and led to a larger release of organic compounds besides ammonia upon HT.
In fact, the search of solvent extracts of hydrothermally treated powders analyzed previously (Pizzarello & Williams 2012) revealed a suite of  numerous branched (methyl-, dimethyl-, ethyl-) pyridines in both CIs and Murchison, but not in other types of meteorites. Some of these N-containing one-ring hydrocarbons, already miscible in water (Merck Index, 10th ed.), could have been more easily released from the polymer upon HT and collected in the ammonia containing water phase. The isotopic composition of the pyridines was not assessed at the time but, were they also enriched in 15N, their HT release would provide a reasonable explanation for the higher overall 15N value found for CI hotspots in this study.
numerous branched (methyl-, dimethyl-, ethyl-) pyridines in both CIs and Murchison, but not in other types of meteorites. Some of these N-containing one-ring hydrocarbons, already miscible in water (Merck Index, 10th ed.), could have been more easily released from the polymer upon HT and collected in the ammonia containing water phase. The isotopic composition of the pyridines was not assessed at the time but, were they also enriched in 15N, their HT release would provide a reasonable explanation for the higher overall 15N value found for CI hotspots in this study.
Figure 3(b) shows the plot of hotspots in  depleted H-IOM versus the 18O compositions of the stones and is linear. CC display a wide range of oxygen isotopic composition between types as a consequence of the interaction of primordial isotopic reservoirs in the solar nebula, subsequent asteroidal processes (Clayton & Mayeda 1999), characterize their minerals and also define their classification or absence of it, such as for TL and Bells. That the IOM 15N composition may correlate so clearly with the
depleted H-IOM versus the 18O compositions of the stones and is linear. CC display a wide range of oxygen isotopic composition between types as a consequence of the interaction of primordial isotopic reservoirs in the solar nebula, subsequent asteroidal processes (Clayton & Mayeda 1999), characterize their minerals and also define their classification or absence of it, such as for TL and Bells. That the IOM 15N composition may correlate so clearly with the  ratios of the meteorites' matrices after the loss of ammonia appears to point to a later acquisition stage for the molecule within the pre-accretionary history of their parent bodies.
ratios of the meteorites' matrices after the loss of ammonia appears to point to a later acquisition stage for the molecule within the pre-accretionary history of their parent bodies.
The data in Table 1 fall within the broad ranges of 15N anomalies reported for solar materials in previous studies, e.g., for a regolith in the metal-rich chondrite Isheyevo that had astounding variations (−310 to +4900‰; Briani et al. 2009), for IDPs from the comet 26P/Grigg–Skjellerup dust stream (+70 to +485‰, for the bulk and +410 to +1400‰ for hotspots; Busemann et al. 2006) and the +480 for the Dragonfly IDP (Messenger 2000).
For meteoritic IOM in particular, its bulk 15N composition was determined for several meteorites by Alexander et al. (2007) and the pertinent values from that study are listed in Table 1. IOM 15N anomalies were also determined by Busemann et al. (2006) for a group of meteorites similar to the ones we studied. The study provided only the single largest average values of the anomalies for each IOM and they were found to wary from +1740 to +2000‰ for three CR2 meteorites, to +410‰ for Tagish Lake and +3200‰ for Bells. Also, a more recent attempt to characterize isolated 15N-rich hotspots for the CO QUE 97416 (an Ornans-type, a thermally altered class of meteorites) found nitriles to be the carrier phase of the anomaly (Bose et al. 2014).
Based on the knowledge and hypotheses available to date on the chemical evolution of biogenic elements, their molecules identified outside the Earth and the mineralogy present in early solar system bodies such as asteroids, three main features of our results cannot be accounted for directly and are in need of further explanation: (1) the presence of freeable ammonia in insoluble materials; (2) the 15N enrichment of the IOM ammonia; and (3) the dependence of IOM ammonia 15N enrichments with the mineralogy of the stones. We argue that the following sequence and/or progression of events relying on plausible solar processes would account for our results.
As presently understood (e.g., Levy 1985), the protosolar nebula formed from a collapsing molecular cloud in the local ISM and formed a nebula with a star at its center, the Sun, while left-over matter containing dust and molecules of the cloud reached different and farther temperature zones in a spinning disk, all the while clumping, condensing and being subjected to sometimes intense radiation, e.g., during the T-Tauri phase (Elmegreen 1979). 15N-enriched ammonia formed during these stages by vacuum UV irradiation of N2 and H2 condensed mixtures. Macromolecular assemblages involving formaldehyde polymerization, both inherited from the cloud and newly formed in cooler regions of the protosolar or solar nebula, readily incorporated the 15N-enriched ammonia in concentrated "hotspots," adsorbed upon and/or intermixed with the mineral precursors present in those regions and was eventually incorporated by assembling small bodies such as comets and asteroids.
Later the asteroids, whose material was left unable to coalesce into a planet under the gravitational tag of earlier-formed giant planets, incorporated 15N-enriched ammonia, both bound and free, and used it after developing a hydrous environment for the formation of 15N-enriched compounds such as amino acids via known reactions (e.g., Pizzarello & Shock 2010). Comets have long been known to contain ammonia (Wyckoff et al. 1991) and recent measurements revealed  N‰ up to ∼+1000, with the authors implying solar nebular fractionation effects to justify the high value (Rousselot et al. 2014; Shinnaka et al. 2014). Gaseous planets such as Titan, where ammonia has an estimated
N‰ up to ∼+1000, with the authors implying solar nebular fractionation effects to justify the high value (Rousselot et al. 2014; Shinnaka et al. 2014). Gaseous planets such as Titan, where ammonia has an estimated  N‰
N‰  +397, may have incorporated this solar ammonia as well (Mandt et al. 2014).
+397, may have incorporated this solar ammonia as well (Mandt et al. 2014).
Recent findings support this scenario. The isotope selective photodissociation of N2 by UV irradiation was assessed theoretically (Heays et al. 2014) and 15N enrichments of ∼12,000‰ were produced experimentally by 90 nm UV radiation of an N2 H2 mix (Chakraborty et al. 2014). As the authors note in the latter reference, the photoionization of N2, H2, and H are 14.534, 15.42, and 13.6 eV, respectively, i.e., greater than the energy used in their experiments, but they point out that the ionization energy of NH3 is 10.2 eV and may lead to photoionization at longer wavelengths, such as those used in these experiments, as well as introduce additional isotope effects. We would assume that the environment of the early nebula, far more complex than experimental conditions and thought to be highly heterogeneous, could have intervened significantly in these reactions. The protosolar disk could contain mixtures of simple molecules such as H2O ice, CH4, CO2, OH, or HCN (e.g., Carr & Najita 2008), which, if exposed to stellar UV photons or cosmic rays breaking atomic bonds, would produce reactive ions and radicals within the ice-rocky matrix. Subsequent to N2 photodissociation, fractionation caused by exchange reactions between atomic 15N and other N-containing ions would form several intermediate 15N-enriched ionization products in the solar nebula:  NH
NH NH
NH NH
NH NH
NH Photochemical self-shielding effects, similar to those observed for O isotopes in CCs' mineral component (Clayton 2002), could also have been responsible for producing N-anomalies in the protosolar nebula and produce 15N-rich ammonia in the presence of appropriate ice-rock mixtures. The temperature in the disk can be as high as 1000 K (Chiang & Goldreich 1997), depending on the proximity to the star and the stage of the star (e.g., the T-Tauri stage), which may be sufficient to help initiate reactions such as the formation of NH2 from NH (NIST data base), however, the majority of other reactions to produce ammonia require low temperatures of ∼10 K temperatures, typical in the outer parts of the solar system or midplane of the protosolar disk.
Photochemical self-shielding effects, similar to those observed for O isotopes in CCs' mineral component (Clayton 2002), could also have been responsible for producing N-anomalies in the protosolar nebula and produce 15N-rich ammonia in the presence of appropriate ice-rock mixtures. The temperature in the disk can be as high as 1000 K (Chiang & Goldreich 1997), depending on the proximity to the star and the stage of the star (e.g., the T-Tauri stage), which may be sufficient to help initiate reactions such as the formation of NH2 from NH (NIST data base), however, the majority of other reactions to produce ammonia require low temperatures of ∼10 K temperatures, typical in the outer parts of the solar system or midplane of the protosolar disk.
As to the incorporation of ammonia into a developing and/or developed formaldehyde-type polymer, experimental polymerization reactions of formaldehyde found ammonia addition to be indispensable to (Schutte et al. 1993) or at least increase its rate of polymerization (Cody et al. 2011). On the basis of more recent data by Kebukawa et al. (2013), who followed the formose polymerization in the laboratory under diverse conditions and detailed its composition by several spectroscopic techniques, the many functional groups predicted throughout such polymer, e.g., carbonyls (R-CO-R'), would have made ammonia addition through its lone pair of electrons ( not only possible but abundant (Breslow 1959; Appayee & Breslow 2014).
not only possible but abundant (Breslow 1959; Appayee & Breslow 2014).
The stability of IOM-bound ammonia during the extensive washes and rinses of the demineralization procedures can also be accounted for by the above experimental findings. For example, the Cody group conducted N-XANES spectroscopy of a formaldehyde polymer and detected nitriles when the polymer was synthesized in the presence of ammonia (Kebukawa et al. 2013). Nitriles will hydrolyze during acid washes but the product carboxamides would be stabilized by CO−=NH+
 resonance, survive demineralization procedures, and release ammonia upon HT; formamide in particular comes to mind (Harada 1967).
resonance, survive demineralization procedures, and release ammonia upon HT; formamide in particular comes to mind (Harada 1967).
Albeit more tentatively, we could also assume that ammonia retention could have benefited from the very complexity of the IOM assemblages. Many functional groups are observed in the IOM by NMR, such as carboxyls (COOH; Cody et al. 2002), and model experiments of formaldehyde polymerization have shown disordered sequences of phenols and units with phenolic rings (Pizzarello et al. 2013); all of these O-containing, highly branched and interlocked assemblages might have helped caging amide groups though hydrogen bonding at the very low pH conditions for isolating the IOM but allowed the release of ammonia upon HT.
4. CONCLUSIONS
The experimental data presented here offer new insights on the possible distributions and molecular evolution of volatile N-containing compounds in the Solar System. The hypothesis we propose also offers a reasonable explanation for the presence of highly 15N-enriched molecules in the IOM, a materials inherited from the ISM, as well as for the chance of these molecules and materials to associate with mineral phases in different zones of the nebula. The interesting isotopic coherence discovered for two CI IOMs also points to the desirability of similar analyses for several meteorites of the same class, as we are planning.
Nitrogen is, after H and the noble gases, one of the most abundant elements in cosmic environment, the Solar System and, on the Earth, a major constituent of the biosphere. The possibility that CC meteorites seeded the Earth with prebiotic precursors has often been offered, however, the caveat raised for these proposals has been the lack of explanation for how an heterogeneous and random mix of abiotic compounds, in the thousands (Schmitt-Kopplin et al. 2010) and minute amounts (μg g−1 at best; Pizzarello & Shock 2010), could have worked their way into the exacting selectivity of even the simpler biochemistry.
That ammonia could be released from the IOMs of meteorites in μg mg−1 (Pizzarello et al. 2011) and the findings that such simple but essential prebiotic molecule can form abundantly in solar environments, be incorporated in asteroids and delivered to early Earth environments, open a new understanding of the prebiotic molecular inventory in our early planet as well as inform our estimates for the possible habitability of other planetary systems.
Funding for this work was provided by the NASA Exobiology and Astrobiology Program, with grant # NNX10AT81G to S.P.. M.B. thanks Dr. Peter Williams (NSF ARRA-960334) and Dr. Rick Hervig (NSF EAR-0948878 & NSF EAR-1352996) for supporting her during this work. S.P. is indebted to Ana L. Moore for fruitful discussions and the ASU Center for Meteorite Studies for providing the indispensable distilled HF solutions. Both authors are sincerely thankful to an anonymous reviewer for a prompt and careful revision.