ABSTRACT
We report on the discovery of methyl acetate, CH3COOCH3, through the detection of a large number of rotational lines from each one of the spin states of the molecule: AA species (A1 or A2), EA species (E1), AE species (E2), and EE species (E3 or E4). We also report, for the first time in space, the detection of the gauche conformer of ethyl formate, CH3CH2OCOH, in the same source. The trans conformer is also detected for the first time outside the Galactic center source SgrB2. From the derived velocity of the emission of methyl acetate, we conclude that it arises mainly from the compact ridge region with a total column density of (4.2 ± 0.5) × 1015 cm−2. The derived rotational temperature is 150 K. The column density for each conformer of ethyl formate, trans and gauche, is (4.5 ± 1.0) × 1014 cm−2. Their abundance ratio indicates a kinetic temperature of 135 K for the emitting gas and suggests that gas-phase reactions could participate efficiently in the formation of both conformers in addition to cold ice mantle reactions on the surface of dust grains.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
The line survey of Orion carried out by Tercero and collaborators using the IRAM 30 m telescope presents a forest of lines arising from isotopologues and vibrationally excited states of abundant species (see, e.g., Tercero et al. 2010, 2011; Daly et al. 2013). The problem of identifying these features was a real challenge as initially we had more than 8000 unidentified lines. The analysis of the data has been done molecule by molecule (Tercero 2012). For each species we explored the literature for spectroscopic information on the isotopologues and vibrationally excited states, but substantial laboratory work was missing for most of these species. In 2006 we began a close collaboration with different spectroscopic laboratories that allowed us to identify nearly 4000 of these unknown lines (often called weeds). The 13C and 15N isotopologues from ethyl cyanide, CH3CH2CN, were measured by Demyk et al. (2007) and Margulès et al. (2009). Several vibrationally excited states of the same species were characterized in the laboratory by Daly et al. (2013). The 13C, deuterated, and 18O isotopologues of methyl formate were observed in the laboratory by Carvajal et al. (2009), Margulès et al. (2010), and Tercero et al. (2012), respectively. The vibrationally excited state ν12 of formamide was measured in the laboratory by Motiyenko et al. (2012). Finally, A. López et al. (in preparation) have characterized in the laboratory several vibrationally excited states of vinyl cyanide, CH2CHCN, which have been identified in Orion together with all its isotopologues 13C, 15N, and D. All these isotopologues and vibrationally excited states were detected in space for the first time, thanks to these new laboratory data. In addition, the study of the survey was divided in the analysis of different families of molecules: CS-bearing species (Tercero et al. 2010), silicon-bearing molecules (Tercero et al. 2011), SO and SO2 (Esplugues et al. 2013). Work on other species, such as HCN, HNC, and HCO+, CH3CN, HC3N, and HC5N, and organic saturated O-rich species, is in progress (N. Marcelino et al., in preparation; T. A. Bell et al., in preparation; G. B. Esplugues et al., in preparation; A. López et al., in preparation).
Although many of the still 4000 remaining U lines could belong to rare isotopologues of complex organic molecules, we believe that we are ready now to begin the search for new molecular species. The study of a cloud such as Orion could provide important clues on the formation of complex organic molecules on the grain surfaces and/or in the gas phase. A systematic line survey with most weeds removed permits us to address the problem of the abundances of isomers and derivates of key species, such as methyl formate, ethyl cyanide, and others. Moreover, it will constitute the best spectral template for future ALMA observations of hot cores.
In this Letter we report the detection for the first time in space of methyl acetate, CH3COOCH3, from the detection of many lines from the states AA, AE, EA, and EE (E3, and E4) of this molecule at 3, 2, and 1.3 mm. The gauche conformer of ethyl formate (an isomer of methyl acetate), for which the anti conformer was previously detected in SgrB2 by Belloche et al. (2009), has also been detected in Orion. This detection of ethyl formate outside the Galactic center indicates that this species is also efficiently produced in hot cores.
2. OBSERVATIONS
The observations were carried out using the IRAM 30 m radiotelescope during several periods (see Tercero et al. 2010). System temperatures were in the range 200–800 K for the 1.3 mm receivers, 200–500 K for the 2 mm receivers, and 100–350 K for the 3 mm receivers, depending on the particular frequency, weather conditions, and source elevation. The intensity scale was calibrated using two absorbers at different temperatures and using the Atmospheric Transmission Model (Cernicharo 1985; Pardo et al. 2001). Pointing and focus were regularly checked on the nearby quasars 0420−014 and 0528+134. Observations were made in the balanced wobbler-switching mode. We pointed the observations toward IRc2 at α(J2000) = 5h35m14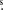 5, δ(J2000)=−5° 22' 30
5, δ(J2000)=−5° 22' 30 0. The data were processed using the IRAM GILDAS software3 (developed by the Institute de Radioastronomie Millimetrique). We considered lines with intensities ⩾0.02 K, covering three or more channels. Figures are shown in units of main beam antenna temperature, TMB =
0. The data were processed using the IRAM GILDAS software3 (developed by the Institute de Radioastronomie Millimetrique). We considered lines with intensities ⩾0.02 K, covering three or more channels. Figures are shown in units of main beam antenna temperature, TMB =  /ηMB, where ηMB is the main beam efficiency. A more detailed description of the observations can be found in Tercero et al. (2010).
/ηMB, where ηMB is the main beam efficiency. A more detailed description of the observations can be found in Tercero et al. (2010).
3. FREQUENCY AND INTENSITY PREDICTIONS FOR METHYL ACETATE
Methyl acetate, [CH3–O–C(=O)–CH3], has been studied by Sheridan et al. (1980) using Stark spectrometers in the frequency region from 8 to 40 GHz. In 2011, the investigation was considerably improved with 315 new lines recorded using the molecular beam Fourier transform microwave spectrometer in Aachen and 519 lines recorded using the free jet absorption Stark-modulated millimeterwave (FJASMmm) spectrometer in Bologna (Tudorie et al. 2011). A newly written program BELGI-Cs-2Tops based on the Hamiltonian described by Ohashi et al. (2004) was used to fit the complete data set with 27 molecular parameters, up to J = 19 and Ka = 7. More than 800 new microwave and millimeter-wave measurements were assigned to the ground-state transitions in methyl acetate and fit, leading to rms deviations of 4 kHz for the microwave lines and of 40 kHz for the millimeter-wave lines, i.e., to residuals essentially equal to the experimental measurement errors. The heights for two internal rotation barriers were determined to be of 102 cm−1 for the acetyl CH3 internal rotor and of 422 cm−1 for the ester CH3. As the theoretical background and code presently used have been extensively described by Tudorie et al. (2011), we will summarize here only the main characteristics.
The BELGI-Cs-2Tops program is restricted to (1) asymmetric top molecules containing two non-equivalent CH3 internal rotors, and (2) molecules belonging to the Cs point group at equilibrium. This code is most closely related to a code used only once in the literature for a treatment of the microwave spectrum of the molecule N-methylacetamide [CH3–NH–C(=O)–CH3] (Ohashi et al. 2004) although some differences exist as detailed in Tudorie et al. (2011).
The torsional and rotational Hamiltonian is diagonalized in separate symmetry blocks, each characterized by one of the five (σ1, σ2) pairs, where σ1 and σ2 designate the symmetry indicators for each of the A and B tops. We follow the notation used in Table 1 of Ohashi et al. (2004), i.e., we have for the five symmetry species: σ1 = 0, σ2 = 0, AA species (A1 or A2 in the G18 permutation-inversion group of the molecule); σ1 = ±1, σ2 = 0, EA species (E1); σ1 = 0, σ2 = ±1, AE species (E2); σ1 = ±1, σ2 = ∓1, EE species (E3); and σ1 = ±1, σ2 = ±1, EE species (E4). The higher barrier hindering the ester methyl group internal rotation (422 cm−1) corresponds to the smaller splittings between the AA and the E2 lines, whereas the lower barrier (102 cm−1) hindering the acetyl methyl group is responsible for the larger splittings between the AA and the E1 lines. The splittings between the E3 lines and the E4 lines are due to the coupling between the two tops. Statistical weights for methyl acetate are 16, 16, 16, 8, and 8 for the A1 or A2 (AA), E1 (AE), E2 (EA), E3, and E4, respectively (Ohashi et al. 2004). The zero-point energies for the J = K = 0 levels are 99.9450 cm−1, 99.9551 cm−1, 101.0947 cm−1, 101.1047 cm−1, and 101.1049 cm−1 for A, E2, E1, E3, and E4 species, respectively.
The spectroscopic constants determined from the previous fit published in Tudorie et al. (2011) are used in the present study to predict the transition frequencies for the νt = 0 torsional ground state up to J = 30. Since we did only fit the observed lines in the laboratory up to J = 19, Ka ⩽ 7, large uncertainties exist for the higher J and Ka values (we estimate 1σ uncertainties of 0.1–0.5 MHz for J < 25 and 0.5–2 for 25 ⩽J ⩽ 30). For the present Letter we also have implemented in the BELGI-Cs-2Tops the calculation of the intensities using the same method as described in Hougen et al. (1994) and Kleiner (2010) for the one-top codes.4 The BELGI-Cs-2Tops code is written in a quasi-principal-axis-method (PAM) axis system which can be obtained by a rotation about the c-axis from the principal axis system (PAM) by the angle θ, as shown in Equation (9) of Ohashi et al. (2004). The value of θ in methyl acetate is determined to be −0.0316385(95) radians (Tudorie et al. 2011). Using the values determined by Sheridan et al. (1980) in the PAM axis system, we obtain for the components of the electric dipole moments in the quasi-PAM axis system, μa = −0.008 D and μb = 1.641 D.
4. RESULTS AND DISCUSSION
The predicted frequencies and intensities, together with the energy of the levels, have been implemented in the MADEX code (Cernicharo 2012). Each one of the five states, AA, AE (E2), EA (E1), EE (E3 and E4), has been considered as an independent molecular species for the calculation of line intensities. For the kinetic temperature of Orion levels above J = 30 could be significantly populated and some lines could also contribute to the Orion spectrum. The predictions from our spectroscopic code have, however, large uncertainties for larger Js (see above). The synthetic spectrum of methyl acetate was calculated assuming LTE conditions (due to the lack of collisional rates for this molecule) for a kinetic temperature of 150 ± 20 K, an LSR velocity of the cloud of 8 km s−1, a line width of 3 km s−1, and a column density for each state AA, EA, and AE of (10.4 ± 1.0) × 1014 cm−2, and of (5.2 ± 1.0) × 1014 cm−2 for the E1 and E2 states. Size (15'') and offset (7'') from the pointing position (IRc2) of the compact ridge component (see Favre et al. 2011; Genzel & Stutzki 1989; Blake et al. 1987) are taken into account in our model. Beam dilution is corrected for each line depending on their frequency. Consequently, the total column density of methyl acetate in Orion is 4.2 ± 0.5 × 1015 cm−2, which includes a correction factor to the partition function computed for TK = 150 K and J ⩽ 30 of 2.6. It includes the torsional excited states (estimated energies) and rotational levels up to J = 65. Figures 1–3 show selected lines from the best-fit synthetic spectrum to the Orion data. The full list of detected lines is provided in Table 1 (electronic version), where we list 215 unblended lines as well as 163 lines moderately blended with other species. No unblended lines of CH3COOCH3 are missing. The detection is fully secure taking into account that the systematic pattern of the lines arising from the different states is always present. Lines from νt = 1 could be also detectable as the vibrational partition function indicates that the population of the νt = 1 levels from the two methyl groups will be a factor 1.5–2 below that of the νt = 0 states. Unfortunately, no accurate predictions are available for the torsionally excited states.
Figure 1. Selected lines of methyl acetate at 3 mm, CH3COOCH3, toward Orion-IRc2. The lines from the different states are identified. The continuous green line corresponds to all lines already modeled in our previous papers (see the text).
Download figure:
Standard image High-resolution imageFigure 2. Selected lines of methyl acetate at 2 mm, CH3COOCH3, toward Orion-IRc2. The lines from the different states are identified. The continuous green line corresponds to all lines already modeled in our previous papers (see the text).
Download figure:
Standard image High-resolution imageFigure 3. Selected lines of methyl acetate at 1.3 mm, CH3COOCH3, toward Orion-IRc2. The lines from the different states are identified. The continuous green line corresponds to all lines already modeled in our previous papers (see the text).
Download figure:
Standard image High-resolution imageTable 1. Detected Lines of CH3COOCH3
| J | Ka | Kc | p | J' |  |
 |
p' | State | Predicted | Error | Eu | Sij μ2 | Observed | Tmb | Blend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq. (MHz) | (MHz) | (K) | Freq. (MHz) | (K) | |||||||||||
| 13 | 0 | 13 | 12 | 1 | 12 | E3 | 82779.635 | .012 | 28.6 | 31.30 | 82780.2 | 0.03 | CH3CH2CN | ||
| 33SO2 | |||||||||||||||
| 13 | 0 | 13 | 12 | 1 | 12 | E4 | 82779.725 | .012 | 28.6 | 31.30 | a | ||||
| 13 | 0 | 13 | 12 | 1 | 12 | EA | 82780.026 | .012 | 28.6 | 31.30 | a | ||||
| 13 | 1 | 13 | 12 | 0 | 12 | E4 | 82808.862 | .012 | 28.6 | 31.30 | 82809.2 | 0.04 | HCOOCH3 | ||
| νt = 1 | |||||||||||||||
| 13 | 1 | 13 | 12 | 0 | 12 | E3 | 82808.881 | .012 | 28.6 | 31.30 | a | ||||
| 13 | 1 | 13 | 12 | 0 | 12 | EA | 82809.267 | .012 | 28.6 | 31.30 | a | ||||
| 13 | 0 | 13 | 12 | 1 | 12 | AE | 82823.351 | .012 | 28.6 | 31.30 | 82823.2 | 0.04 | |||
| 13 | 0 | 13 | 1 | 12 | 1 | 12 | 1 | AA | 82823.702 | .012 | 28.6 | 31.30 | a |
Notes. Emission lines of CH3COOCH3 present in the spectral scan of Orion KL from the IRAM 30 m radiotelescope. Columns 1–8 indicate the line transition, Column 9 the state of the molecule, Column 10 the predicted frequency in the laboratory, Column 11 the uncertainty of frequency predictions, Column 12 the upper level energy, Column 13 the line strength, Column 14 the observed frequency assuming a vLSR of 8 km s−1, Column 15 the mean beam temperature, and Column 16 the blends. aBlended with previous line.
Only a portion of this table is shown here to demonstrate its form and content. A machine-readable version of the full table is available.
Download table as: DataTypeset image
Ethyl formate has two conformers, trans (also called anti) and gauche, with the latter being 65 ± 21 cm−1 (94 K) above in energy (Riveros & Wilson 1967). The gauche conformer has two possible orientations of the terminal methyl group and, hence, it could be twice as abundant as the trans conformer if the energy difference were zero (Belloche et al. 2009). We have used the spectroscopic laboratory data from Riveros & Wilson (1967), Meyer & Wilson (1970), and Demaison et al. (1984) to derive the rotational constants which were incorporated into the MADEX code. The predictions were checked against the corresponding entry in the JPL catalog (Pickett et al. 1998). The trans conformer of ethyl formate was detected in SgrB2 by Belloche et al. (2009) but only upper limits were obtained for the gauche one. We have identified both conformers in the line survey of Orion through 90 features free of blending (52 trans and 38 gauche). Selected identified lines are shown in Figure 4. We found that the gauche conformer has less intense lines than the trans one for transitions at 3 and 2 mm. However, at higher frequencies, there are many multiplet transitions of the gauche that coincide in frequency and they become prominent in the Orion data. The best fit to the data (assuming the same physical conditions and source parameter—vLSR, Δv, size, and offset—than those used for methyl acetate corresponding to the compact ridge component of Orion KL) provides a rotational temperature of 150 ± 20 K, and a column density for each conformer of (4.5 ± 1.0) × 1014 cm−2, i.e., a total column density for ethyl formate ≃5 times below that of methyl acetate. From the observed abundance ratio between the trans and gauche conformers, Ngauche/Ntrans ≃ 1 = 2 × e−94/T, we derive a kinetic temperature for the emitting gas of 135 ± 30 K. If the lowest energy conformer of ethyl formate, the trans one, was formed on the ices before the warm phase of the cloud, isomerization to the gauche form will require a high kinetic energy and, perhaps, a long time to overpass the barrier to isomerization of 550 K (Riveros & Wilson 1967). Our result points toward a fast equilibrium between both conformers at 150 K and in a time comparable to the duration of the present warm phase of the cloud. If the molecule is formed in the gas phase, the energy liberated in the process could help in the isomerization process and the observed conformer temperature will reflect the kinetic temperature of the gas. However, no chemical paths are included in the present chemical models to form either ethyl formate or methyl acetate. New laboratory experiments are needed to understand the way these species could be formed in gas phase or in ices and how conformers of the same species can equilibrate at a temperature close to the kinetic temperature of the gas.
Figure 4. Selected lines of the trans (red line) and gauche (blue) conformers of ethyl formate, CH3CH2OCOH, toward Orion-IRc2. The synthetic spectrum corresponds to the same column density for both conformers (see the text). The continuous green line corresponds to all lines already modeled in our previous papers (see the text).
Download figure:
Standard image High-resolution imageMethyl acetate is probably formed via multiple reaction pathways from species detected in hot cores such as methyl formate, acetic acid, and methanol. In icy grain mantles, methanol is one of the common molecular components. Acetic acid is a relatively large molecule that would be difficult to detect in the ice by IR observations and that could be the precursor of methyl acetate and ethyl formate. Only formic acid (HCOOH) has been proposed as a possible carrier of the 7.24 μm band toward high-mass protostars, while the 7.41 μm band could be due to the formate ion (HCOO−) and acetaldehyde (CH3CHO), according to Schutte et al. (1999). Formation of HCOOH on a surface occurs experimentally at low temperatures, mainly through hydrogenation of the HO–CO complex (Ioppolo et al. 2011). Other possible formation routes in the ice are via precursor cations (Woon 2011) or by reactions of superthermal O(3P) atoms and CH4 with an overcoat of CO (Madzunkov et al. 2010). Also photon or electron irradiation of H2O:CO ice mixtures leads to formation of formic acid among other products, including methanol in the case of photoprocessing (Watanabe et al. 2007; Bennett et al. 2011). HCOOH was found to spontaneously deprotonate when sufficient water is present to stabilize charge transfer complexes. Both ammonia and water can serve as proton acceptors, yielding ammonium (NH ) and hydronium (H3O+) counterions (Park & Woon 2006). The so-formed formate ion (HCOO−) might intervene in the formation of species like methyl acetate in the ice matrix, but this was, to our knowledge, not confirmed experimentally. Brouillet et al. (2013) have recently observed with high angular resolution CH3OCH3 and CH3OCOH toward Orion-IRc2 and conclude that the similarity in the spatial distribution of both species points toward a common precursor. The observation of a similar abundance for the two conformers of ethyl formate points to a gas-phase production path rather than to a low-temperature ice formation mechanism. Radicals such as methoxy (CH3O; Cernicharo et al. 2012) or CH3CO could play an important role in the gas-phase chemistry. However, methoxy has been observed only toward cold dark clouds (Cernicharo et al. 2012) and it is not detected in our line survey of Orion; CH3CO has not yet been detected in the Interstellar Medium (ISM).
) and hydronium (H3O+) counterions (Park & Woon 2006). The so-formed formate ion (HCOO−) might intervene in the formation of species like methyl acetate in the ice matrix, but this was, to our knowledge, not confirmed experimentally. Brouillet et al. (2013) have recently observed with high angular resolution CH3OCH3 and CH3OCOH toward Orion-IRc2 and conclude that the similarity in the spatial distribution of both species points toward a common precursor. The observation of a similar abundance for the two conformers of ethyl formate points to a gas-phase production path rather than to a low-temperature ice formation mechanism. Radicals such as methoxy (CH3O; Cernicharo et al. 2012) or CH3CO could play an important role in the gas-phase chemistry. However, methoxy has been observed only toward cold dark clouds (Cernicharo et al. 2012) and it is not detected in our line survey of Orion; CH3CO has not yet been detected in the Interstellar Medium (ISM).
The two conformers of ethyl formate and methyl acetate are isomers of the C3H6O2. Three additional isomers, propanoic (propionic) acid (CH3CH2COOH), hydroxyacetone (CH3COCH2OH), and methoxyacetaldehyde (CH3OCH2OH), could be also present in Orion. The three species are implemented in MADEX. For hydroxyacetone, the available spectroscopic data have been summarized by Braakman et al. (2010) and cover frequencies up to 431.8 GHz (dipole moments from Kattija-Ari & Harmony 1980). We obtain an upper limit to its column density of 8 ×1013 cm−2 (see also Apponi et al. 2006 for an upper limit to its column density toward SgrB2). For propionic acid and methoxyacetaldehyde, we also obtain upper limits to their column densities of 1.6 × 1014 and 2 × 1014 cm−2, respectively. We note, however, that frequency predictions above 40 GHz for these two molecular species are rather uncertain (Stifvater 1975; Ouyang & Howard 2008; Hirano et al. 1987). Hence, of the known possible non-cyclic isomers of C3H6O2, methyl acetate appears to be the most abundant one. Laboratory spectroscopic data are needed for propionic acid and methoxyacetaldehyde in order to draw further conclusions on their contribution to the ice mantle and gas-phase chemistry of hot cores, and to the forest of still unknown spectral features in Orion.
J. Cernicharo, B. Tercero, G. Muñoz Caro, and A. López thank the Spanish MICINN for funding support through grants, AYA2009- 07304, AYA2011-29375, and CSD2009-00038. I. Kleiner acknowledges support from the French national PCMI program, and support from the ANR-08-BLAN-0054 contract (France). J. Cernicharo thanks U. Paris Est for an invited professor position associated to this work.
Footnotes
- *
This work was based on observations carried out with the IRAM 30 m telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain).
- 3
- 4
The BELGI-Cs code is publicly available at the PROSPE website, Programs for ROtational SPEctroscopy´´, managed by Kisiel at http://www.ifpan.edu.pl/~kisiel/prospe.htm.







