-
PDF
- Split View
-
Views
-
Cite
Cite
Rochelle P. Walensky, A. David Paltiel, Elena Losina, Lauren M. Mercincavage, Bruce R. Schackman, Paul E. Sax, Milton C. Weinstein, Kenneth A. Freedberg, The Survival Benefits of AIDS Treatment in the United States, The Journal of Infectious Diseases, Volume 194, Issue 1, 1 July 2006, Pages 11–19, https://doi.org/10.1086/505147
Close - Share Icon Share
Abstract
BackgroundAs widespread adoption of potent combination antiretroviral therapy (ART) reaches its tenth year, our objective was to quantify the cumulative survival benefits of acquired immunodeficiency syndrome (AIDS) care in the United States
MethodsWe defined eras corresponding to advances in standards of human immunodeficiency virus (HIV) disease care, including opportunistic infection prophylaxis, treatment with ART, and the prevention of mother-to-child transmission (pMTCT) of HIV. Per-person survival benefits for each era were determined using a mathematical simulation model. Published estimates provided the number of adult patients with new diagnoses of AIDS who were receiving care in the United States from 1989 to 2003
ResultsCompared with survival associated with untreated HIV disease, per-person survival increased 0.26 years with Pneumocystis jiroveci pneumonia prophylaxis alone. Four eras of increasingly effective ART in addition to prophylaxis resulted in per-person survival increases of 7.81, 11.05, 11.57, and 13.33 years, compared with the absence of treatment. Treatment for patients with AIDS in care in the United States since 1989 yielded a total survival benefit of 2.8 million years. pMTCT averted nearly 2900 infant infections, equivalent to 137,000 additional years of survival benefit
ConclusionsAt least 3.0 million years of life have been saved in the United States as a direct result of care of patients with AIDS, highlighting the significant advances made in HIV disease treatment
In the face of the global AIDS pandemic, advancement in the treatment of HIV infection has been striking, but this progress has been associated with substantial economic costs. In 2006, US federal governmental agencies will allocate $21 billion to HIV/AIDS activities [1]. This includes ∼$3 billion for HIV/AIDS research at the National Institutes of Health; $12.6 billion for treatment under Medicare and Medicaid and for Ryan White Care Act funds; $2.7 billion for global contributions; nearly $2 billion for cash and housing assistance; and almost $1 billion for prevention activities at the Centers for Disease Control and Prevention (CDC) and local health departments [1]
On 7 December 1995, the first protease inhibitor (PI), saquinavir, was approved by the US Food and Drug Administration, leading to the advent of highly active antiretroviral therapy (ART) for HIV disease. In light of the 10-year anniversary of this important breakthrough in HIV care, we sought to measure what 2 decades of medical research, patient care, and financial investment have produced in terms of overall survival gains. We employed a model-based approach, conducting repeated analyses to explore the clinical consequences of alternative patient-care-innovation pathways. Our objective was to quantify clinical progress in AIDS care, in terms of years of life saved as a result of advances in HIV therapies in the United States
Methods
Analytic overviewWe defined 6 distinct eras of HIV/AIDS treatment from 1989 to 2003, reflecting advances in opportunistic infection (OI) prophylaxis and increasingly effective ART over time (figure 1) [2–12 ]. Using CDC surveillance and other published data, we estimated the number of patients with AIDS who received their diagnoses and entered care each year in the United States [13–15 ]. We used a previously developed computer simulation model of HIV disease to assess per-person survival gains for each treatment era, compared with survival in the absence of treatment [16–18 ]. Cumulative survival estimates for all patients who entered care were then calculated
Timeline of major HIV interventions and when they became the standard of care in the United States. Six treatment eras were defined to correspond to the availability of improved therapies and changing standards of clinical care. The first 2 eras denote advances in opportunistic infection prevention, with prophylaxis for Pneumocystis jiroveci pneumonia (PCP) starting in 1989 and prophylaxis for Mycobacterium avium complex (MAC) disease starting in 1993 [2–4 ]. Treatment with combination antiretroviral therapy (ART) was divided into 4 eras. ART 1 (1996–1997) marks the introduction of potent combination ART with the widespread use of protease inhibitor (PI)–based therapy [5]. ART 2 (1998–1999) includes the sequential use of nonnucleoside reverse transcriptase inhibitor–based regimens followed by PI-based unique regimens [6, 7]. From 2000 to 2002 (ART 3), 3 effective regimen options were available, with increased options for salvage through the use of resistance testing and ritonavir-boosted PIs [8, 10]. ART 4 (2003) included improved drug efficacy as reflected by increased tolerability, decreased regimen complexity, and the introduction of enfuvirtide [9–12 ]. We also considered 2 eras for the prevention of mother-to-child transmission (pMTCT): (1) zidovudine (ZDV) monotherapy alone, 1994–1999, and (2) combination ART, 2000 to present
We also included 2 eras of maternal treatment for the prevention of mother-to-child transmission (pMTCT) of HIV (figure 1). Using CDC data on the number of HIV-infected women in the United States, as well as published birth, transmission, and survival rates for HIV-infected pediatric patients, we derived the number of infant infections averted and translated those into years of life saved [13, 19, 20]
This analysis was conservative; when assumptions were necessary, they were designed to underestimate the total survival benefit. For example, we limited the size of the eligible patient population to those with AIDS; we excluded the early benefits of antiretroviral mono- and dual-drug therapy when survival benefits were more limited and HIV RNA data were not available; we utilized lower estimates for rates of linkage to care; we assumed that those who did not access care in the first year of their AIDS diagnosis were never eligible; and we omitted any benefits of reduced HIV transmission from care. Sensitivity analyses were performed to examine the stability of the results in the face of alternative assumptions regarding delays in the adoption of clinical guidelines, mean CD4 cell count at presentation, the number of patients entering care, and ART efficacies
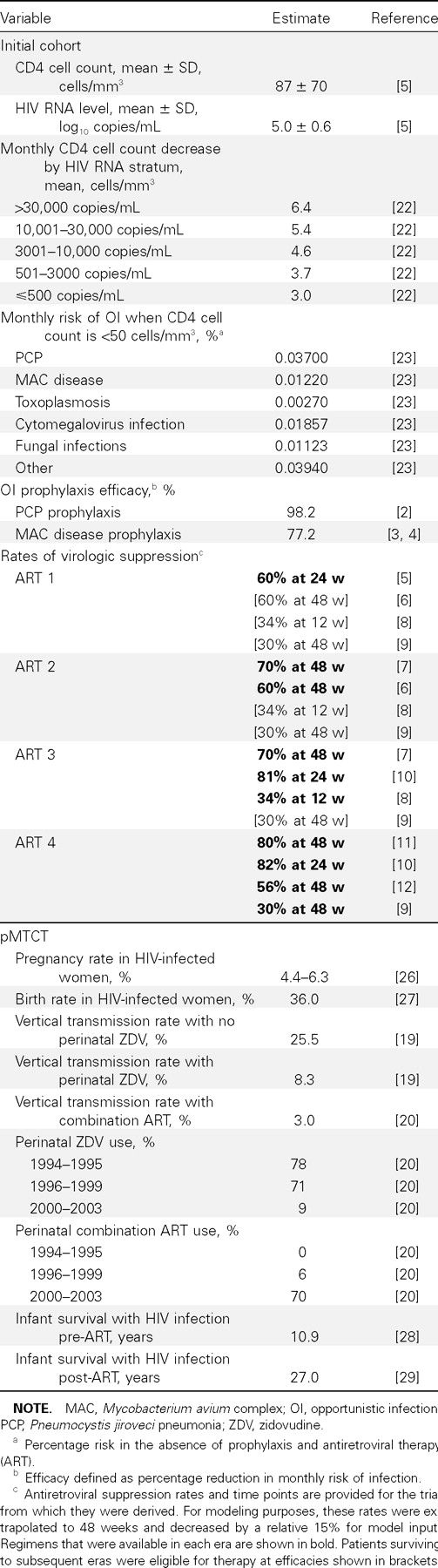
Estimation of the sample populationEligibility for therapy in any treatment era was limited to patients with CDC-defined AIDS; patients with non-AIDS HIV infection were excluded. Recognizing that not all patients with AIDS diagnoses receive timely care, we used national samples to estimate the proportion of patients entering care each year. National data suggest that 88% of eligible patients in the pre-ART era were receiving OI prophylaxis and that 57% of those in all of the ART eras were receiving appropriate comprehensive care [14, 15]. We characterized the newly diagnosed cohort as reflecting patients with advanced AIDS, with a mean CD4 cell count of 87 cells/mm3 (SD, 70 cells/mm3) and a mean HIV RNA level of 5.0 log copies/mL [5]
HIV disease modelThe Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model was used to estimate per-person survival benefits. CEPAC is a widely published computer-based state-transition simulation model of HIV disease that incorporates CD4 cell count; HIV RNA level; ART efficacy; OI incidence, treatment, and prophylaxis; and other important clinical information [16–18 , 21]. “State transition” means that the model characterizes the progression of disease in an individual patient as a sequence of transitions from one “health state” to another
Health states are defined along dimensions that are both descriptive of a patient’s current well-being and predictive of future clinical events. These dimensions include CD4 cell count (>500, 301–500, 201–300, 101–200, 51–100, and ⩽50 cells/mm3) and HIV RNA level (>30,000, 10,001–30,000, 3001–10,000, 501–3000, and ⩽500 copies/mL) [22]. In the model, the level of HIV RNA determines the rate of CD4 cell count decline, and the absolute CD4 cell count governs the monthly risk of OIs and death [16–18 , 22]
In the model, HIV-infected patients are at risk for OIs (Pneumocystis jiroveci pneumonia [PCP], toxoplasmosis, Mycobacterium avium complex [MAC] disease, disseminated fungal infection, cytomegalovirus infection, and bacterial and other infections) on the basis of their CD4 cell count [16, 23]. Patients receive the recommended standards of care in the year of their diagnosis, including regular CD4 cell count and HIV RNA laboratory tests and prophylaxis for PCP and MAC disease [24]
The model is able to accommodate a range of assumptions regarding the number of sequential lines of available ART, as well as their sequencing and efficacy. In the ART eras, patients whose CD4 cell count is <200 cells/mm3 and are in care are eligible for ART. ART functions to suppress the HIV RNA level, producing a concomitant increase in CD4 cell count [5, 7, 8, 16]. The proportion of patients achieving virologic suppression while receiving each regimen is based on rates reported in the clinical trials and adjusted for lower suppression rates observed in nonclinical trial populations [5–12 ]. ART failure is defined as either virologic (an observed increase in HIV RNA level over 2 consecutive months) or clinical (the development of an OI). On failure of therapy, a subsequent regimen is initiated until all regimens available in that era are exhausted; the last line of therapy is continued after failure for its independent effect on averting OIs [25]. Rates of virologic suppression for ART are era specific, as described below
Treatment with combination ART was divided into 4 eras. ART 1 (1996–1997) marks the introduction of potent combination ART with the widespread use of PI-based therapy [5]. ART 2 (1998–1999) includes the sequential use of nonnucleoside reverse-transcriptase inhibitor (NNRTI)–based regimens followed by PI-based unique regimens [6, 7]. From 2000 to 2002 (ART 3), 3 effective regimen options were available, with increased options for salvage through the use of resistance testing and ritonavir-boosted PIs [8, 10]. ART 4 (2003) included improved drug efficacy, as reflected by increased tolerability, decreased regimen complexity, and the introduction of enfuvirtide [9–12 ]. The literature-reported regimen suppression rates used for each ART era are provided in table 1
Data for model inputs and prevention of mother-to-child transmission (pMTCT) estimations
Patients who initiate treatment in one era become eligible for additional therapies later, if they survive to subsequent eras. Reflecting diminishing ART efficacy with increasing ART experience, these later regimens may differ in their suppression rates from those that would be available to patients who initiate therapy in the subsequent era
Hypothetical patients with AIDS enter the model one at a time and are followed until death. A cohort of 1 million patients was simulated, to obtain stable results; summary statistics for the analysis included average numbers of OIs and per-person life expectancy. For each year of AIDS diagnosis from 1989 to 2003, one cohort was simulated with no treatment and the second was simulated with all treatment interventions available during that era; the 2 cohorts’ average life expectancies were then compared, to obtain the net treatment benefit for the cohort that received diagnoses in that year. Survival curves generated from the model were validated against CDC-reported survival curves [13]
Model input dataDetailed data for model input are provided in table 1 [2–12 , 19, 20, 22, 23, 26–29 ]. Briefly, OI prophylaxis efficacy is based on published data and is defined as the percentage reduction in the monthly risk of OIs: 98.2% for PCP and 77.2% for MAC disease [2–4 ]. Rates of virologic suppression for ART are era specific, both for the initial treatment regimen and for the subsequent regimens that are available after drug resistance or poor adherence causes the initial regimen to fail. For example, data for those patients who receive diagnoses in ART 1 (or before then, if they are still alive at the beginning of ART 1) are derived from a trial of a triple-combination regimen that reported HIV RNA suppression in 60% of patients at 24 weeks [5]. This efficacy is reflective of a cohort of patients, all of whom were pretreated with zidovudine alone [5]. Among those still alive by ART 2, efficacy is derived from a second-line efavirenz-based regimen in patients pretreated with nucleoside reverse-transcriptase inhibitors (NRTIs), which achieves 60% suppression at 48 weeks [6]. For those who remain alive in ART 3 and ART 4, third- and fourth-line treatment options become available, with efficacy derived from trials showing suppression rates of 34% at 12 weeks and 30% at 48 weeks, respectively [8, 9]. All reported efficacies are extrapolated to 48 weeks, as reported elsewhere [17]. Because the reported treatment efficacies of available regimens are from clinical trials and may overstate clinical cohort efficacies, we reduced the 48-week efficacy of suppression for each regimen by a factor of 15%. This relative reduction in efficacy represents reported differences in suppression efficacies between an observational Medicaid cohort and a clinical trial population receiving a comparable NNRTI-based regimen at a similar disease stage [11, 30]
pMTCTTo estimate survival gains attributable to pMTCT, the number of HIV-infected women in the United States was obtained from CDC surveillance data [13]. Birth rates; rates of ART utilization; HIV vertical transmission rates with no treatment (25.5%), with peripartum zidovudine treatment (8.3%), and with combination ART (3.0%); and life expectancies for HIV-infected infants are provided in table 1 [19, 20, 26, 27]. Children born HIV negative were assigned age- and race-adjusted life expectancies based on US life tables [31]
Results
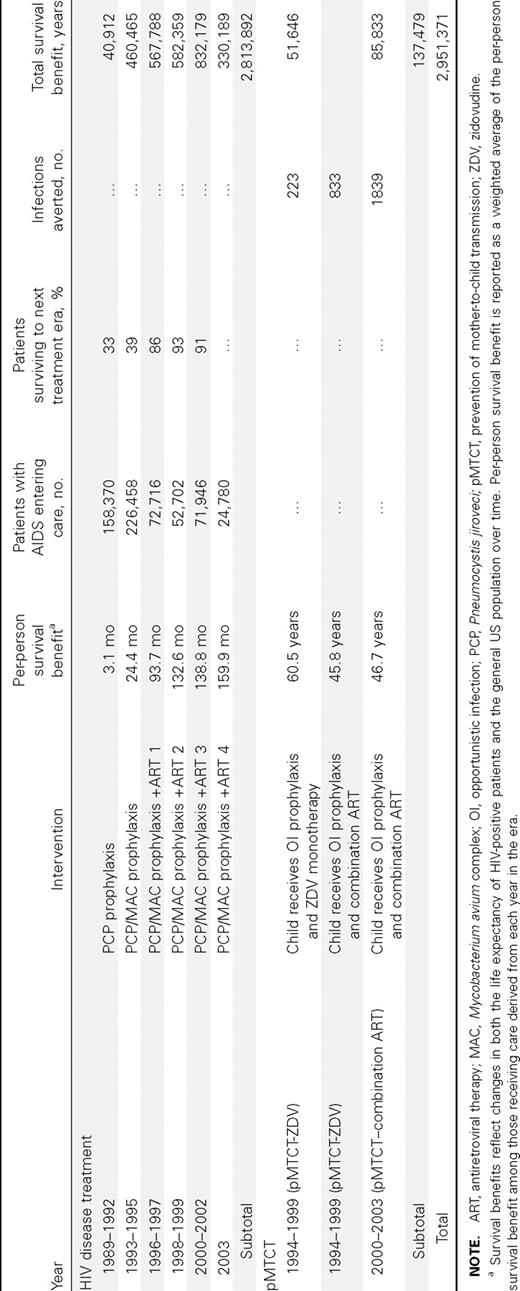
Per-person HIV disease treatment survival benefits among those receiving careResults from the era of PCP prophylaxis alone show that mean per-person life expectancy increased by 3.1 months, compared with that in the absence of prophylaxis (table 2). In this era, 33% of patients survived to 1993 (the PCP and MAC disease prophylaxis era), but only 2% survived to 1996 to receive any highly active ART. Although the life expectancy benefit anticipated from PCP and MAC disease prophylaxis alone was just 2.6 months, the era of PCP and MAC disease prophylaxis led to a mean survival increase of 24.4 months. This increase is largely a result of 39% of this cohort surviving to 1996 and then benefiting from ART 1. PCP and MAC disease prophylaxis combined with ART 1, 2, and 3 resulted in mean per-person survival increases of 93.7, 132.6, and 138.8 months, respectively. By ART 4 (2003), comprehensive AIDS care resulted in a projected per-person survival gain of 159.9 months, or 13.3 years
Per-person survival benefits, numbers of patients with AIDS entering care, and era-specific and cumulative survival benefits
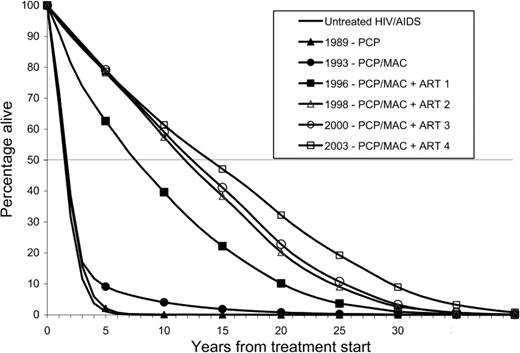
HIV disease treatment survival benefitsThe number of patients entering care in the United States ranged from a maximum of 75,486 per year in the PCP and MAC disease prophylaxis era (1993–1995) to a minimum of 24,780 in ART 4 (2003). The total survival benefit ranged from 40,912 years in the PCP prophylaxis era to 832,179 years in ART 3. The cumulative survival benefit for AIDS-related OI prophylaxis and combination ART was 2,813,892 years. Of these, 1,184,851 years have already been realized, and 1,629,041 years are being accrued by current patients with AIDS in care. Model-based survival curves for patients who received diagnoses in the first year of each of the 6 treatment eras (1989, 1993, 1996, 1998, 2000, and 2003) illustrate the improvement in AIDS-associated survival in the United States over time (figure 2). Median survival was 1.7 years for the PCP era, 7.5 years for ART 1, and 14.1 years for ART 4
Survival curve produced by model simulations of the cohort that received diagnoses in the first year of each treatment era, with a mean age at treatment start of 39 years (SD, 9 years). ART, antiretroviral therapy; MAC, Mycobacterium avium complex; PCP, Pneumocystis jiroveci pneumonia
pMTCT survival benefitspMTCT produced notable survival benefits as well. In the zidovudine-alone era (1994–1999), perinatal zidovudine treatment averted 1056 infant infections (table 2). After 2000, when combination ART became widely used in pregnant women, 1839 infant infections were averted. Mean per-child survival gains for the averted infections ranged from 60.5 years if the child was born before 1996 (before combination ART) to 45.8 years during 1996–1999, when combination ART was available. pMTCT led to a survival benefit of 137,479 years (table 2)
The cumulative survival benefit of AIDS-related OI prophylaxis, combination ART, and pMTCT in the United States was 2,951,371 years
Sensitivity analysesSeveral sensitivity analyses were performed to examine the stability of the results. When we assumed that the changes in standards of care might take 1 year to implement (as may occur for patients treated in lower-volume centers), total survival benefits decreased to 2.4 million years. When we examined the impact of alternative, less widely accepted estimates of the rate of vertical transmission associated with ART [32], estimated survival benefits due to pMTCT fell from 137,479 to 99,680 years
We also relaxed the conservative estimates in sensitivity analyses. When we used the full reported efficacy of ART from the clinical trials [11, 30], per-person survival gains in ART 4 increased from 159.9 to 188.8 months, and total survival gains increased by 500,000 years. When we assumed that linkage to care was not 57% but as high as 76% [15], survival gains increased by 710,000 years. As a surrogate for earlier presentation to care, a healthier AIDS cohort—with a mean CD4 cell count of 175 cells/mm3—was also examined, since, after 1992, ∼70% of new AIDS diagnoses were made according to a CD4 cell count criterion of <200 cells/mm3 alone [33]. Cumulative survival benefits in this scenario increased by 740,000 years. Simultaneously relaxing all of these conservative assumptions resulted in a total survival benefit of >5.2 million years
Discussion
We utilized national surveillance data, efficacy data, and a state-transition probability model to estimate the survival benefits of AIDS therapy in the United States. This type of analysis does not lend itself readily to traditional forms of scientific investigation, since there is no counterfactual to the history of AIDS treatment in reality. Projected per-person survival after an AIDS diagnosis increased from 19 months (1.6 years) in the absence of treatment to 179 months (14.9 years) by 2003, a gain of 160 months (13.3 years). This survival benefit greatly exceeds that achieved for patients with many other chronic diseases in the United States [34–42 ]. Although this type of analysis has not been conducted for many diseases, we have synthesized data from the literature to estimate life-expectancy gains for patients with other severe and chronic diseases (figure 3). For example, chemotherapy for non–small-cell lung cancer results in an average survival benefit of 7 months, and bone marrow transplantation for relapsed non-Hodgkins lymphoma is associated with a survival benefit of 92 months [34, 41, 42]. Early in the course of the pandemic, OI prophylaxis had a profound impact on changing the face of AIDS and on shaping the perception that HIV disease was treatable [43]. However, the current magnitude of the life-expectancy gain from AIDS treatment is largely attributable to combination ART; this has transformed AIDS from a fatal disease to a highly treatable chronic condition, with average survival from AIDS diagnosis that is now >14 years
Per-person survival gains for patients with various interventions for chronic diseases in the United States [34–42 ]. Opportunistic infection prophylaxis (OI proph) confers a 3-month benefit (if no benefit to antiretroviral therapy [ART] in those eras is assumed). ART produces 160 months of per-person survival gain by the year 2003. BMT, bone marrow transplant; MI, myocardial infarction
As many as 312,000 of the estimated 1,185,000 people infected with HIV in the United States are thought to be unaware of their serostatus [44]. Of those who are aware of their infection, only 57% are estimated to be in care [15]. Using a cohort of patients with higher CD4 cell counts as a surrogate for earlier diagnosis and linkage to care, we found that an additional 740,000 years of life might have been saved, had all patients with AIDS in the United States received appropriate treatment on diagnosis. Thus, our findings not only demonstrate the striking survival gains achieved via advances in AIDS treatment but also emphasize the importance of expanded HIV testing and linkage to care, so that greater numbers of infected persons can access lifesaving therapy
Previous work using the same HIV disease model estimated that, in 1996, ART led to per-person survival benefits of 18.5 months [16]. Updated results from the current analysis suggest that mean survival increases from the same era (ART 1) were 93.7 months. The additional 75.2 months reflects the value of new HIV therapies that have been developed and are available for patients receiving failing regimens. Our results are also consistent with those demonstrating the effectiveness of current ART in large cohort studies, in which the median AIDS-associated survival was found to be extended by 14.8 years [45]
This analysis has several limitations. Patients entering ART 1 often were mono-drug– or dual-drug–therapy experienced. We addressed this problem by modeling early treatment efficacy rates on the basis of patients who had received zidovudine monotherapy [5]. To match estimates in clinical cohorts rather than clinical trials, we decreased the reported efficacy rates from all trials by an additional 15% [11, 30]. Estimating the year of entry into care compared with the year of AIDS diagnosis was a challenge; we conservatively limited the eligible patients entering care each year, reduced their assumed state of health (mean CD4 cell count, 87 cells/mm3), and assumed that those who did not access care in the year of diagnosis were never eligible for care
The analysis did not account for later ART-related toxicities that may result in, for example, cardiac disease or diabetes [46]. This exclusion is unlikely to have had a major impact on the analysis. Although the relative risk of cardiac disease may be increased as a result of ART, this increase is greatly outweighed by the absolute reduction in the risk of AIDS-related complications from ART [47]. Previous work estimating HIV treatment–induced changes in lipid levels suggests that hyperlipidemia reduces overall life expectancy by ∼1 month [48]
Early reports of OI prophylaxis and ART efficacy in less-developed countries have suggested that the per-person survival gains in these settings may be comparable to those in the United States, even in the absence of customized, highly monitored care [49]. With 38 million HIV-infected people worldwide, the increased availability of ART through the WHO-sponsored “3 by 5” initiative, as well as through the President’s Emergency Plan for AIDS Relief (PEPfAR) and other sources, has the potential to save hundreds of millions of years of life in the global setting and speaks to the imperative to deliver treatment to individuals in these countries quickly and efficiently [50, 51]
HIV disease has claimed >20 million lives worldwide and more than half a million lives in the United States alone [13, 51]. Our analysis demonstrates the striking scientific and clinical benefits achieved in the fight against this disease. Ten years after the introduction of potent combination ART, at least 3 million years of life have been saved in the United States
References
(See the editorial commentary by Vermund, on pages 1–5.)
Presented in part: 12th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 22–25 February 2005 (abstract 143LB)
Potential conflicts of interest: P.E.S. receives consultancy funding, teaching honoraria, and grant support from the following sources: Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Gilead, Pfizer, and GlaxoSmithKline. All other authors report no potential conflicts of interest
Financial support: National Institute of Allergy and Infectious Diseases (grants K23AI01794, K24AI062476, K25AI50436, R01AI42006, and P30AI060354); National Institute of Mental Health (grant R01MH65869); National Institute on Drug Abuse (grants K01DA017179 and R01DA015612); US Centers for Disease Control and Prevention (grant S1396-20/21)




![Timeline of major HIV interventions and when they became the standard of care in the United States. Six treatment eras were defined to correspond to the availability of improved therapies and changing standards of clinical care. The first 2 eras denote advances in opportunistic infection prevention, with prophylaxis for Pneumocystis jiroveci pneumonia (PCP) starting in 1989 and prophylaxis for Mycobacterium avium complex (MAC) disease starting in 1993 [2–4 ]. Treatment with combination antiretroviral therapy (ART) was divided into 4 eras. ART 1 (1996–1997) marks the introduction of potent combination ART with the widespread use of protease inhibitor (PI)–based therapy [5]. ART 2 (1998–1999) includes the sequential use of nonnucleoside reverse transcriptase inhibitor–based regimens followed by PI-based unique regimens [6, 7]. From 2000 to 2002 (ART 3), 3 effective regimen options were available, with increased options for salvage through the use of resistance testing and ritonavir-boosted PIs [8, 10]. ART 4 (2003) included improved drug efficacy as reflected by increased tolerability, decreased regimen complexity, and the introduction of enfuvirtide [9–12 ]. We also considered 2 eras for the prevention of mother-to-child transmission (pMTCT): (1) zidovudine (ZDV) monotherapy alone, 1994–1999, and (2) combination ART, 2000 to present](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/194/1/10.1086_505147/2/m_194-1-11-fig001.jpeg?Expires=1716312419&Signature=Y46VS78mNXif0v7k6Mz1lOTyVdBzYiJ9Akf5hAIQn8qk6ZSB0nbWN3HMv-Jqg0wS10UdO5plHcj-5gdUjXb0o~H1s8xFIigkp25dITCVoivT-yPjZpBEOsLUfx2NsRNfesOmbw8e0jFCkxoErt3PibwHCDPxLy2HkauWwnR~qiLrNQp0S9bLUNeRJd~zYwccRgy2AuFlH-3-TppnZQ5LWSMjs5T61AAMe2kEKp5k~J2Ef14fw8V6uz0L~PBPGI0mrbPJwxiN6v~9MvOmBTvbkTDgymeFNnAD16vJnFyTmTuTyx754~pE~nMpkdDO0~~YFeIVc39HSIQ2HVMVT430OA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Per-person survival gains for patients with various interventions for chronic diseases in the United States [34–42 ]. Opportunistic infection prophylaxis (OI proph) confers a 3-month benefit (if no benefit to antiretroviral therapy [ART] in those eras is assumed). ART produces 160 months of per-person survival gain by the year 2003. BMT, bone marrow transplant; MI, myocardial infarction](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/194/1/10.1086_505147/2/m_194-1-11-fig003.jpeg?Expires=1716312419&Signature=h5JKMi9yhBPgZURzWsCRoNRGMKLUi1VkH0pNtcIfEfSl-w4z4p7JTZ9rEGX-ZDfplnG9JCuRveSEC1Vy8jeATbEdbT8Hr7EFbLfYR9WNOVwWHw0PYyUZrLDuV-15IOx8h-CxpYF6-Q3OhGpbQMjDYluH~F9tunVLOnSBD0Zv2dV0fJxc7SGCjgInCTcduswEkS082EI2-uaLc54luEON7CX2mAjAKBSagoY0HmdTYKi~7fws9Dwd2yZ2VkXtA34ZZK2JET9nRb8ijX8HrW5XLmqNNRvWrbDh7lDl6Mz7mt~c2wmXLpaWP3tIj6dBMSexTz2Gy1J0tdYnvQM2WziUag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
