Abstract
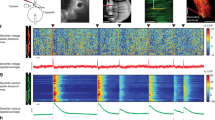
Low-voltage activated (LVA) Ca2+ currents have been characterized in a large variety of neurons including cerebellar Purkinje cells (PCs). This review summarizes and discusses the biophysical, pharmacological properties, as well as the molecular identity of LVA Ca2+ channels described in PCs in various experimental conditions. Putative functional roles for LVA Ca2+ currents include generation of low-threshold Ca2+ spikes (LTS) that underlie burst firing, promotion of intrinsic oscillatory behaviour, Ca2+ entry close to the resting membrane potential and synaptic potentiation. Based on our recent findings on cerebellar rat PCs in slice cultures, this review presents the major evidence demonstrating that LVA Ca2+ channels produce a dendritic initiated LTS with a regulated propagation to the soma. This new role for LVA Ca2+ channels is particularly important in determining firing patterns in PCs.
Similar content being viewed by others
References
Johnston D, et al. Active properties of neuronal dendrites. Annu Rev Neurosci 1996; 19: 165–186.
Yuste R, Tank DW. Dendritic integration in mammalian neurons, a century after Cajal. Neuron 1996; 16: 701–716.
Llinas R, et al. Dendritic spikes and their inhibition in alligator Purkinje cells. Science 1968; 160(832): 1132–1135.
Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 1980; 305: 197–213.
Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol 1980; 305: 171–195.
Snutch TP, Reiner PB. Ca2+ channels: diversity of form and function. Curr Opin Neurobiol 1992; 2: 247–253.
Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 1996; 58: 329–348.
Perez-Reyes E, et al. Molecular characterization of a neuronal lowvoltage-activated T-type calcium channel. Nature 1998; 391(6670): 896–900.
Cribbs LL, et al. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res 1998; 83: 103–109.
Soong TW, et al. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 1993; 260(5111): 1133–1136.
Bourinet E, et al. The alpha 1E calcium channel exhibits permeation properties similar to low-voltage-activated calcium channels. J Neurosci 1996; 16: 4983–4993.
Lacinova L, Klugbauer N, Hofmann F. Regulation of the calcium channel alpha(1G) subunit by divalent cations and organic blockers. Neuropharmacology 2000; 39: 1254–1266.
Liljelund P, Netzeband JG, Gruol DL. L-Type calcium channels mediate calcium oscillations in early postnatal Purkinje neurons. J Neurosci 2000; 20: 7394–7403.
Usowicz MM, et al. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron 1992; 9: 1185–1199.
Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron 1992; 9: 85–95.
Westenbroek RE, et al. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron 1992; 9: 1099–1115.
Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci 1991; 11: 2259–2269.
Mintz IM, et al. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature 1992; 355(6363): 827–829.
Hillman D, et al. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci USA 1991; 88: 7076–7080.
Bossu JL, Fagni L, Feltz A. Voltage-activated calcium channels in rat Purkinje cells maintained in culture. Pflugers Arch 1989; 414: 92–94.
Bossu JL, Dupont JL, Feltz A. Calcium currents in rat cerebellar Purkinje cells maintained in culture. Neuroscience 1989; 30: 605–617.
Crepel F, Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol 1986; 372: 1–23.
Hirano T, Hagiwara S. Kinetics and distribution of voltage-gated Ca, Na and K channels on the somata of rat cerebellar Purkinje cells. Pflugers Arch 1989; 413: 463–489.
McDonough SI, Bean BP. Mibefradil inhibition of T-type calcium channels in cerebellar Purkinje neurons. Mol Pharmacol 1998; 54: 1080–1087.
Kaneda M, et al. Low-threshold calcium current in isolated Purkinje cell bodies of rat cerebellum. J Neurophysiol 1990; 63: 1046–1051.
Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 1999; 19: 1663–1674.
Mouginot D, Bossu JL, Gahwiler BH. Low-threshold Ca2+ currents in dendritic recordings from Purkinje cells in rat cerebellar slice cultures. J Neurosci 1997; 17: 160–170.
Bindokas VP, Brorson JR, Miller RJ. Characteristics of voltage sensitive calcium channels in dendrites of cultured rat cerebellar neurons. Neuropharmacology 1993; 32: 1213–1220.
Watanabe S, et al. Differential roles of two types of voltage-gated Ca2+ channels in the dendrites of rat cerebellar Purkinje neurons. Brain Res 1998; 791(1–2): 43–55.
Volsen SG, et al. The expression of neuronal voltage-dependent calcium channels in human cerebellum. Brain Res Mol Brain Res 1995; 34: 271–282.
Pouille F, et al. Dendro-somatic distribution of calcium-mediated electrogenesis in purkinje cells from rat cerebellar slice cultures. J Physiol 2000; 527 Pt 2: 265–282.
Yokoyama CT, et al. Biochemical properties and subcellular distribution of the neuronal class E calcium channel alpha 1 subunit. J Neurosci 1995; 15: 6419–6432.
Talley EM, et al. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 1999; 19: 1895–1911.
Craig PJ, et al. Distribution of the voltage-dependent calcium channel alpha 1G subunit mRNA and protein throughout the mature rat brain. Eur J Neurosci 1999; 11: 2949–2964.
Chemin J, et al. Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca2+ channel gating properties. Biophys J 2001; 80: 1238–1250.
Monteil A, et al. Molecular and functional properties of the human alpha(1G) subunit that forms T-type calcium channels. J Biol Chem 2000; 275: 6090–6100.
Kostyuk PG. Low-voltage activated calcium channels: achievements and problems. Neuroscience 1999; 92: 1157–1163.
Karst H, Joels M, Wadman WJ. Low-threshold calcium current in dendrites of the adult rat hippocampus. Neurosci Lett 1993; 164(1–2): 154–158.
Destexhe A, et al. Dendritic low-threshold calcium currents in thalamic relay cells. J Neurosci 1998; 18: 3574–3588.
Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci USA 1994; 91: 5207–5211.
Gruol DL, Deal CR, Yool AJ. Developmental changes in calcium conductances contribute to the physiological maturation of cerebellar Purkinje neurons in culture. J Neurosci 1992; 12: 2838–2848.
Nam SC, Hockberger PE. Analysis of spontaneous electrical activity in cerebellar Purkinje cells acutely isolated from postnatal rats. J Neurobiol 1997; 33: 18–32.
Dumont EC, et al. Physiological properties of central amygdala neurons: species differences. Eur J Neurosci 2002; 15: 545–552.
Cavelier P, et al. Control of the propagation of dendritic lowthreshold Ca2+ spikes in Purkinje cells from rat cerebellar slice cultures. J Physiol 2002; 540(Pt 1): 57–72.
Miyasho T, et al. Low-threshold potassium channels and a lowthreshold calcium channel regulate Ca2+ spike firing in the dendrites of cerebellar Purkinje neurons: a modeling study. Brain Res 2001; 891(1–2): 106–115.
Tarasenko AN, et al. Two types of low-voltage-activated Ca2+ channels in neurones of rat laterodorsal thalamic nucleus. J Physiol 1997; 499(Pt 1): 77–86.
Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci 1996; 16: 5567–5582.
Chemin J, et al. Specific contribution of human T-type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol 2002; 540(Pt 1): 3–14.
Tottene A, Moretti A, Pietrobon D. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci 1996; 16: 6353–6363.
Kavalali ET, et al. Dendritic Ca2+ channels characterized by recordings from isolated hippocampal dendritic segments. Neuron 1997; 18: 651–663.
Lee JH, et al. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alphalH. Biophys J 1999; 77: 3034–3042.
Zamponi GW, Bourinet E, Snutch TP. Nickel block of a family of neuronal calcium channels: subtype- and subunit-dependent action at multiple sites. J Membr Biol 1996; 151: 77–90.
Nakashima YM, et al. Properties of Ba2+ currents arising from human alpha 1E and alpha 1E beta3 constructs expressed in HEK293 cells: physiology, pharmacology, and comparison to native T-type Ba2+ currents. Neuropharmacology 1998; 37: 957–972.
Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology 1997; 36: 879–893.
Newcomb R, et al. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry 1998; 37: 15353–15362.
Tottene A, Volsen S, Pietrobon D. Alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci 2000; 20: 171–178.
Stuart G, Hausser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron 1994; 13: 703–712.
Wilson MA, et al. GENESIS: a system for simulating neural networks. In: Touretzky D, editor. Advances in Neural Information Processing Systems. San Mateo, CA: Morgan Kaufmann, 1989: 485–492.
De Schutter E, Bower JM. An active membrane model of the cerebellar Purkinje cell. I. Simulation of current clamps in slice. J Neurophysiol 1994; 71: 375–400.
Eccles JC, Ito M, Szentagotha I. The Cerebellum as a Neuronal Machine. Berlin: Springer-Verlag, 1967.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavelier, P., Bossu, JL. Dendritic low-threshold Ca2+ channels in rat cerebellar Purkinje cells: Possible physiological implications. Cerebellum 2, 196–205 (2003). https://doi.org/10.1080/14734220310016141
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220310016141