Abstract
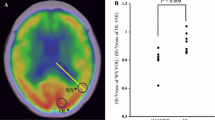
Human T-cell lymphotropic virus (HTLV)-1 is associated with a chronic progressive neurologic disease known as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) that affects 0.2% to 3% of HTLV-1-infected people. The authors aimed at exploring, in vivo, whether brain volume reduction occurs in patients with HAM/TSP through the use of magnetic resonance imaging (MRI). T1 pre/postcontrast spin echo-weighted images (WIs) and T2WIs of the brain were obtained in 19 HAM/TSP patients and 14 age- and sex-matched healthy volunteers. Both patients and healthy individuals were imaged at a 1.5-Tesla magnet by employing a conventional head coil. Focal T1 and T2 abnormalities were calculated and two measurements of brain parenchyma fraction (BPF) were obtained by using SIENAx (Structural Image Evaluation,using Normalisation, of Atrophy; University of Oxford, Oxford, UK) and MIPAV (Medical Image Processing, Analysis, and Visualization; National Institutes of Health, Bethesda, USA) from T1WIs. No significant differences in BPF were found between patients and healthy subjects when using either SIENAx or MIPAV. Analysis of individual patients detected that BPF was lower by 1 standard deviation (SD) relative to patients’ average BPF in one patient. The authors conclude that reductions in BPF do not occur frequently in patients with HAM/TSP. However, the authors believe that one individual case of significant brain atrophy raises the question as to whether atrophy selectively targets the spinal cord of HAM/TSP patients or may involve the brain as well. A larger patient population analyzing regional brain volume changes could be helpful in determining whether brain atrophy is a marker of disease in patients with HAM/TSP.
Similar content being viewed by others
References
Aye MM, Matsuoka E, et al (2000). Histopathological analysis of four autopsy cases of HTLV-1-associated myelopathy/tropical spastic paraparesis: inflammatory changes occur simultaneously in the entire central nervous system. Acta Neuropathol (Berl) 100: 245–252.
Bagnato F, Butman JA, et al (2005). Conventional magnetic resonance imaging features in patients with tropical spastic paraparesis. J NeuroVirol, 11: 525–534.
Cruickshank JK, Rudge P, et al (1989). Tropical spastic paraparesis and human T cell lymphotropic virus type 1 in the United Kingdom. Brain 112: 1057–1090.
Fukushima T, Ikeda T, et al (1994). Cognitive event-related potentials and brain magnetic resonance imaging in HTLV-1 associated myelopathy (HAM). J Neurol Sci 126: 30–39.
Godoy AJ, Kira J, et al (1995). Characterization of cerebral white matter lesions of HTLV-I-associated myelopathy/tropical spastic paraparesis in comparison with multiple sclerosis and collagen-vasculitis: a semiquantitative MRI study. J Neurol Sci 133: 102–111.
Howard AK, Li DK, et al (2003). MRI contributes to the differentiation between MS and HTLV-I associated myelopathy in British Columbian coastal natives. Can J Neurol Sci 30: 41–48.
Jacobson S (2002). Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J Infect Dis 186 (Suppl 2), S187-S192.
Kannagi M, Ohashi T, et al (2004). Immunological risks of adult T-cell leukemia at primary HTLV-I infection. Trends Microbiol 12: 346–352.
Kasahata N, Shiota J, et al (2003). Acute human T-lymphotropic virus type 1-associated myelopathy: a clinicopathologic study. Arch Neurol 60: 873–876.
Kurtzke JF (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33: 1444–1452.
Melo A, Moura L, et al (1993). Magnetic resonance imaging in HTLV-I associated myelopathy. Arq Neuropsiquiatr 51: 329–332.
Miller DH, Barkhof F, et al (2002). Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain 125: 1676–1695.
Osame M (1990). Review of WHO Kagoshima Meeting and Diagnostic guidelines for HAM/TSP. In Human Retrovirology HTLV 1st ed. Blattner WA (ed.): New York, Raven Press pp 191–197.
Pelletier D, Garrison K, et al (2004). Measurement of whole-brain atrophy in multiple sclerosis. J Neuroimaging 14: 11S-19S.
Pham DL, Xu C, et al (2000). Current methods in medical image segmentation. Annu Rev Biomed Eng. 2: 315–337.
Rudick RA, Fisher E, et al (1999). Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 53: 1698–1704.
Rusinek H, Endo Y, et al (2004). Atrophy rate in medial temporal lobe during progression of Alzheimer disease. Neurology 63: 2354–2359.
Shattuck DW, Sandor-Leahy SR, et al (2001). Magnetic resonance image tissue classification using a partial volume model. NeuroImage 13: 856–876.
Smith SM, De Stefano N, et al (2001). Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr 25: 466–475.
Smith SM, Zhang Y, et al (2002). Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 17: 479–489.
Takenouchi N, Yao K, et al (2004). Immunopathogensis of HTLV-I associated neurologic disease: molecular, histopathologic, and immunologic approaches. Front Biosci 9: 2527–2539.
Yamano Y, Nagai M, et al (2002). Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 99: 88–94.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the Intramural Research Program of the NINDS, NIH.
Rights and permissions
About this article
Cite this article
Griffith, C., Bagnato, F., Gupta, S. et al. Brain volume measurements in patients with human T-cell lymphotropic virus-1-associated tropical spastic paraparesis. Journal of NeuroVirology 12, 349–355 (2006). https://doi.org/10.1080/13550280600941665
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280600941665