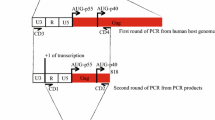
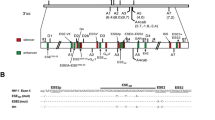
Abstract
Alternative splicing of the JC Virus (JCV) precursor early mRNA yields five transcripts that encode proteins that regulate the life cycle of this human polyomavirus. Large T protein (TAg) mediates viral DNA replication and oncogenic activities, and small t protein influences these functions under certain conditions. Recently, three new early proteins, T′135,T′136, andT′165, were discovered that contain sequences overlapping amino-terminal TAg functional domains. Initial studies with the T′ proteins suggested they contribute to viral DNA replication and transformation. Mutation of a donor splice site utilized by all three T′ mRNAs creates a mutant that exhibits a 10-fold decrease in viral DNA replication compared to wild type JCV. To assess the influence that individual T′ proteins have on the replication process, a set of T′ acceptor site mutants was created in which the unique second acceptor splice site of each T′ mRNA was altered to eliminate production of one, two or all three T′ mRNAs. The patterns of early mRNA and protein expression in these seven mutants were examined, and it was found that mutation of the T′135 acceptor site resulted in the utilization of cryptic splice sites and the generation of new T′ species. Additional mutations were made to prevent these aberrant splicing reactions prior to measuring DNA replication potential of the mutants. DpnI assays revealed that each T′ protein contributes to TAg-mediated DNA replication activity. The three single mutants that express two T′ proteins and the double mutant that only produces T′136, exhibited levels of replication equivalent to that of wild type virus, whereas the two double mutants that fail to express T′136 replicated about twofold less efficiently than wild-type JCV. Replication activity of the triple acceptor site mutant, like that of the T′ donor site mutant from an earlier study, was impaired significantly.
Similar content being viewed by others
References
Aebi M, Hornig H, Padgett RA, Reiser J, Weissmann C (1986). Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell 47: 555–565.
Bikel I, Mamon H, Brown EL, Boltax J, Agha M, Livingston DM (1986). The t-unique coding domain is important to the transformation maintenance function of the simian virus 40 small t antigen. Mol Cell Biol 6: 1172–1178.
Bollag B, Chuke W-F, Frisque RJ (1989). Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J Virol 63: 863–872.
Bollag B, Prins C, Snyder EL, Frisque RJ (2000). Purified JC virus T and T′ proteins differentially interact with the retinoblastoma family of tumor suppressor proteins. Virology 274: 165–178.
Brodsky JL, Pipas JM (1998). Polyomavirus T antigens: molecular chaperones for multiprotein complexes. J Virol 72: 5329–5334.
Campbell KS, Mullane KP, Aksoy IA, Stubdal H, Zalvide J, Pipas JM, Silver PA, Roberts TM, Schaffhausen BS, DeCaprio JA (1997). DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Gene Dev 11: 1098–1110.
Cegielska A, Moarefi I, Fanning E, Virshup DM (1994a). T-antigen kinase inhibits simian virus 40 DNA replication by phosphorylation of intact T antigen on serines 120 and 123. J Virol 68: 269–275.
Cegielska A, Shaffer S, Derua R, Goris J, Virshup DM (1994b). Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol Cell Biol 4: 4616–4623.
Chuke W-F, Walker DL, Peitzman LB, Frisque RJ (1986). Construction and characterization of hybrid polyomavirus genomes. J Virol 60: 960–971.
Dornreiter I, Höss A, Arthur AK, Fanning E (1990). SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J 9: 3329–3336.
Elliot DJ (2000). Splicing and the single cell. Histol Histopathol 15: 239–249.
Fanning E (1992). Simian virus-40 large T-antigen—the puzzle, the pieces, and the emerging picture. J Virol 66: 1289–1293.
Frisque RJ (1983). Regulatory sequences and virus-cell interactions of JC virus. In: Polyomaviruses and human neurological disease. Sever JL, Madden DL (ed). New York: Alan R. Liss, Inc, pp 41–59.
Frisque RJ (1999). JC and BK viruses (Papovaviridae). In: Encyclopedia of Virology. Granoff A, Webster RG, (eds). Academic Press: London, pp 876–883.
Frisque RJ, Bream GL, Cannella MT (1984). Human polyomavirus JC virus genome. J Virol 51: 458–469.
Frisque RJ, White FA III (1992). The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. In: Molecular neurovirology. Roos RP (ed). Totowa, NJ: Humana Press, pp 25–158.
Haggerty S, Walker DL, Frisque RJ (1989). JC virus-simian virus 40 genomes containing heterologous regulatory signals and chimeric early regions: identification of regions restricting transformation by JC virus. J Virol 63: 2180–2190.
Hirt B (1967). Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol 26: 365–369.
Krawczak M, Reiss J, Cooper DN (1992). The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet 90: 41–54.
Lopez AJ (1998). Alternative splicing of pre-mRNA: developmental consequences and mechanisms of regulation. Annu Rev Genet 32: 279–305.
Lynch KJ, Frisque RJ (1990). Identification of critical elements within the JC virus DNA replication origin. J Virol 64: 5812–5822.
Lynch KJ, Haggerty S, Frisque RJ (1994). DNA replication of chimeric JC virus-simian virus 40 genomes. Virology 204: 819–822.
Molin M, Akusjärvi G (2000). Overexpression of essential splicing factor ASF/SF2 blocks the temporal shift in adenovirus pre-mRNA splicing and reduces virus progeny formation. J Virol 74: 9002–9009.
Mount SM (1982). A catalogue of splice junction sequences. Nucleic Acids Res 10: 459–472.
Noble JCS, Pan ZQ, Prives C, Manley JL (1987). Splicing of SV40 early pre-mRNA to large T and small t mRNAs utilizes different patterns of lariat branch sites. Cell 50: 227–236.
Padgett RA, Konarska MM, Aebi M, Hornig H, Weissman C, Sharp PA (1985). Nonconsensus branch-site sequences in the in vitro splicing of transcripts of mutant rabbit beta-globin genes. Proc Natl Acad Sci USA 82: 8349–8353.
Pipas JM (1992). Common and unique features of T-antigens encoded by the polyomavirus group. J Virol 66: 3979–3985.
Prives C (1990). The replication functions of SV40 T antigen are regulated by phosphorylation. Cell 61: 735–738.
Reed R, Maniatis T (1985). Intron sequences involved in lariat formation during pre-mRNA splicing. Cell 41: 95–105.
Ruskin B, Greene JM, Green MR (1985). Cryptic branch point activation allows accurate in vitro splicing of human beta-globin intron. Cell 41: 833–844.
Scheidtmann KH, Mumby MC, Rundell K, Walter G (1991a). Dephosphorylation of simian virus-40 large-T antigen and p53 protein by protein phosphatase-2A— inhibition by small-T antigen. Mol Cell Biol 11: 1996–2003.
Scheidtmann KH, Virshup DM, Kelly TJ (1991b). Protein phosphatase-2A dephosphorylates simian virus-40 large T-antigen specifically at residues involved in regulation of DNA-binding activity. J Virol 65: 2098–2101.
Sheng Q, Denis D, Ratnofsky M, Roberts TM, DeCaprio JA, Schaffhausen B (1997). The DnaJ domain of polyomavirus large T is required to regulate RB family tumor suppressor function. J Virol 71: 9410–9416.
Smale ST, Tjian R (1986). T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol 6: 4077–4087.
Sock E, Enderich J, Wegner M (1999). The J domain of papovaviral large tumor antigen is required for synergistic interaction with the POU-domain protein Tst-1/Oct6/SCIP. Mol Cell Biol 19: 2455–2464.
Sock E, Wegner M, Fortunato EA, Grummt F (1993). Large T-antigen and sequences within the regulatory region of JC virus both contribute to the features of JC virus DNA replication. Virology 197: 537–548.
Sompayrac LM, Danna KJ (1981). Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci USA 78: 7575–7578.
Stillman B (1994). Smart machines at the replication fork. Cell 78: 725–728.
Stojdl DF, Bell JC (1999). SR protein kinases: the splice of life. Biochem Cell Biol 77: 293–298.
Sullivan CS, Cantalupo P, Pipas JM (2000a). The molecular chaperone activity of simian virus 40 large T antigen is required to disrupt Rb-E2F family complexes by an ATP-dependent mechanism. Mol Cell Biol 20: 6233–6243.
Sullivan CS, Tremblay JD, Fewell SW, Lewis JA, Brodsky JL, Pipas JM (2000b). Species-specific elements in the large T-antigen J domain are required for cellular transformation and DNA replication by simian virus 40. Mol Cell Biol 20: 5749–5757.
Swenson JJ, Frisque RJ (1995). Biochemical characterization and localization of JC virus large T antigen phosphorylation domains. Virology 212: 295–308.
Swenson JJ, Trowbridge PW, Frisque RJ (1996). Replication activity of JC virus large T antigen phosphorylation and zinc finger domain mutants. J Neurovirol 2: 78–86.
Tevethia SS, Epler M, Georgoff I, Teresky A, Marlow M, Levine AJ (1992). Antibody response to human papovavirus JC (JCV) and simian virus-40 (SV40) T-antigens in SV40 T antigen-transgenic mice. Virology 190: 459–464.
Trowbridge PW, Frisque RJ (1993). Analysis of G418-selected Rat2 cells containing prototype, variant, mutant, and chimeric JC-virus and SV40 genomes. Virology 196: 458–474.
Trowbridge PW, Frisque RJ (1995). Identification of three new JC virus proteins generated by alternative splicing of the early viral mRNA. J Neuro Virol 1: 195–206.
Wegner M, Drolet DW, Rosenfeld MG (1993). Regulation of JC virus by the POU-domain transcription factor Tst-1: implications for progressive multifocal leukoencephalopathy. Proc Natl Acad Sci USA 90: 4743–4747.
Weisshart K, Taneja P, Jenne A, Herbig U, Simmons DT, Fanning E (1999). Two regions of simian virus 40 T antigen determine cooperativity of double-hexamer assembly on the viral origin of DNA replication and promote hexamer interactions during bidirectional origin DNA unwinding. J Virol 73: 2201–2211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prins, C., Frisque, R.J. JC virus T′ proteins encoded by alternatively spliced early mRNAs enhance T antigen-mediated viral DNA replication in human cells. Journal of NeuroVirology 7, 250–264 (2001). https://doi.org/10.1080/13550280152403290
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/13550280152403290