Summary
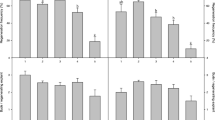
Two independent experiments were performed to establish micropropagation of Cleome spinosa from stem segments. In the first experiment, direct shoot organogenesis on hypocotyl explants from 2-mo.-old nursery-grown seedlings was obtained on Murashige and Skoog medium with different combinations of benzyladenine (BA) and 6-furfurylaminopurine, added either individually or in combination. Best proliferation rates occurred in the presence of 2.2 and 4.4 μM BA and the highest mean number of shoots was produced in response to 4.4 μM BA. In the second experiment, regeneration via direct organogenesis was also obtained from nodal and internodal segments of axenic plants cultured in the presence of BA (4.4 and 8.8 μM) in association with indole-3-acetic acid (IAA) (0.57 and 1.14 μM). Internodal explants were the most responsive on all media tested. The best mean number of shoots per explant was achieved on medium with 4.4 μM BA in association with 0.57 μM IAA. Histological studies of the globular structures formed at the apical portion of the explants revealed direct shoot regeneration and adventitious shoot differentiation from meristematic centers around the vascular bundles of the primary regenerants. All shoots elongated and rooted on MS0 medium. The acclimatization rates ranged between 70 and 84%. Plants reached to maturity and flowered 4 mo. after transfer to ex vitro conditions.
Similar content being viewed by others
References
Ahmad, V. U.; Qazi, S.; Zia, N. B.; Xu, C.; Clardy J. Cleocarpone, a triterpenoid from Cleome brachycarpa. Phytochemistry 29(2):670–672; 1990.
Ahmed, Z. F.; Rizk, A. M.; Hammouda, F. M.; Seif El-Nasr, M. M. Naturally occurring glucosinolates with special reference to those of family Capparidaceae. Planta Med. 21(2):35–60; 1972.
Beena, M. R.; Martin, K. P.; Kurti, P. B.; Hariharan, M. Rapid in vitro propagation of medicinally important Ceropegia candelabrum. Plant Cell Tiss. Organ Cult. 72:285–289; 2003.
Bobak, M.; Blehová, A.; Kristín, J.; Ovecka, M.; Samaj, J. Direct plant regeneration from leaf explants of Drosera rotundifolia cultured in vitro. Plant Cell Tiss. Orgna Cult. 43:43–49; 1995.
Brassard, N.; Brissette, L.; Lord, D.; Laliberte, S. Elongation, rooting and acclimatization of micropropagated shoots from mature material of hybrid larch. Plant Cell Tiss. Organ Cult. 44:37–44; 1996.
Brummit, R. K. Vascular plants families and genera. Kew: Royal Botanic Gardens; 1992.
Collins, D. O.; Reynolds, W. F.; Reese, P. B. New Cembranes from Cleome spinosa. J. Nat. Prod. 67:179–183; 2004.
Das, P. C.; Patra, A.; Mandal, S.; Mallick, B.; Das, A.; Chatterjee, A. Cleogynol, a novel dammarane triterpenoid from Cleome gynandra. J. Nat. Prod. 62:616–618; 1999.
Deora, N. S.; Shekhaway, N. S. Micropropagation of Capparis decidua (Forsk.) Edgew.—a tree of arid horticulture. Plant Cell Rep. 15:278–281; 1995.
Eapen, S.; Tivarekar, S.; George, L. Thidiazuron-induced shoot regeneration in pigeonpea (Cajanus cajan L.). Plant Cell Tiss. Organ Cult. 53:217–220; 1998.
Fushiya, S.; Kishi, Y.; Hattori, K.; Batkhuu, J.; Takano, F.; Singad, A. N. B. Okuyama, T. Flavonoids from Cleome droserifolia suppres NO production in activated macrophages in vitro. Planta Med. 65:404–407; 1999.
George, E. F. Plant propagation by tissue culture. London: Exegetics Ltd.; 1996.
Germano, M. P.; Pasquale, R.; D’Angelo, V.; Catania, S.; Silvari, V.; Costa, C. Evaluation of extracts and isolated fraction Capparis spinosa L. buds as an antioxidant source. J. Agric. Food Chem. 50:1168–1171; 2002.
Girija, S.; Ganapathi, A.; Vengadesan, G. Micropropagation of Crossandra infundibuliformis (L.) Ness. Sci. Hortic. 82:331–337; 1997.
Hashem, F. A.; Wahba, H. E. Isothiocyanates in myrosinase treated herb extract of Cleome chrysantha Decne. and their antimicrobial activities. Phytother. Res. 14(4):284–287; 2000.
Hiregoudar, L. V.; Ashouk, H. G.; Murthy, H. N. In vitro culture of Feronia limonia (L.) swingle from hypocotyls and internodal explants. Biol. Plant. 49(1):41–45; 2005.
Johansen, S. Plant microtechnique. New York: Mc Graw-Hill Book Company; 1940.
Kelkar, S. M.; Krishnamurthy, K. V. Adventitious shoot regeneration from root, internode, petiole and leaf explants of Piper colubrinum link. Plant Cell Rep. 17:721–725; 1998.
Kneifel, W.; Czech, E.; Kopp, B. Microbial contamination of medicinal plants—a review. Planta Med. 68:5–15; 2002.
Liu, C. Z.; Murch, S. J.; El-Demerdash, M.; Saxena, P. K. Artemisia judaica L: micropropagation and antioxidant activity. J. Biotechnol. 110:63–71; 2004.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Murch, S. J.; Liu, C. Z.; Romero, R. M.; Saxena, P. K. In vitro culture and temporary immersion bioreactor production of Crescentia cujete. Plant Cell Tiss. Organ Cult. 78(1):63–68; 2004.
Nagaya, H.; Tobita, Y.; Nagae, T.; Itokawa, H.; Takeya, K.; Halim, A. F.; Abdel-Halim, O. Cytotoxic triterpenes from Cleome africana. Phytochemistry 44(6):1115–1119; 1997.
Nassem, M.; Jha, K. K. Differentiation and regeneration in Cleome leaves culture in vitro. Egypt. J. Bot. 1:37–49; 1994.
Nassem, M.; Jha, K. K. Rapid clonal multiplication of Cleome gynandra DC, through tissue culture. Phytomorphology 47(4):405–411; 1997.
Parimaladevi, B.; Boominathan, R.; Mandal, S. C. Studies on analgesic activity of Cleome viscosa in mice. Fitoterapia 74:262–266; 2003.
Pattnaik, S. K.; Chand, P. K. Rapid clonal propagation of three mulberries, Morus cathayana Hemdl., M. ilhou Koiz. and M. serrata Roxb., through in vitro culture of apical shoot buds and nodal explants from mature trees. Plant Cell Rep. 16:503–508; 1997.
Reddy, P. S.; Rama Gopal, G.; Lakshmi Sita, G. In vitro multiplication of Gymnema sylvestre R.Br.—an important medicinal plant. Curr. Sci. 75(8):843–845; 1998.
Rodríguez, R.; Rey, M.; Cuozzo, L.; Ancora, G. In vitro propagation of caper (Capparis spinosa L.). In Vitro Cell Dev. Biol. Plant 26:531–536; 1990.
Roeser, K. R. Die Nadel des Schwarzkiefer. Massenprodukt und Kunstwerk der Natur. Mikrokosmos 61(2):33–36; 1972.
Selloum, L.; Arrar, L.; Medani, B.; Khenchouche, A.; Bisker, H. Effect of Cleome arabica leaves extract on inflammatory cell response in rat. Biochem. Soc. Trans. 23(4):609; 1995.
Selloum, L.; Sebihi, L.; Mekhalfia, A.; Mahdadi, R.; Senator, A. Antioxidant activity of Cleome arabica leaves extract. Biochem. Soc. Trans. 25:608; 1997.
Sharma, P. K.; Tyagi, P.; Sharma, K. C.; Kothari, S. L. Clonal micropropagation of Crataeva adansonii (DC.) Prodr.: a multipurpose tree. In Vitro Cell Dev. Biol. Plant 39(2):156–160; 2003.
Sharma, V.; Padhya, M. A. In vitro rapid multiplication and propagation of Crataeva nurvala. Indian J. Exp. Biol. 34:243–246; 1996.
Simões, C.; Santos, A. S.; Albarello, N.; Figueiredo, S. F. L. Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). J. Plant Biotechnol. 6(3):199–204; 2004.
Tang, W.; Guo, Z. C. In vitro propagation of loblolly pine via direct somatic organogenesis from mature cotyledons and hypocotyls. Plant Growth Regul. 33(1):25–33; 2001.
Tyagi, P.; Kothari, S. L. Micropropagation of Capparis decidua through in vitro shoot proliferation on nodal explants of mature tree and seedling explants. J. Plant Biochem. Biotechnol. 6:19–23; 1997.
Tyagi, P.; Kothari, S. L. Continuous shoot production for micropropagation of Capparis decidua—a tree of arid agroforestry system., J. Indian Bot. Soc. 80:5–8; 2001.
Vanisree, M.; Lee, C. Y.; Lo, S. F.; Nalawade, S. M.; Lin, C. Y.; Tsay, H. S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 45:1–22; 2004.
Walia, N.; Sinha, S.; Babbar, S. B. Micropropagation of Crataeva nurvala. Biol. Plant. 46(2):181–185; 2003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albarello, N., Simões, C., Rosas, P.F.G. et al. In vitro propagation of Cleome spinosa (Capparaceae) using explants from nursery-grown seedlings and axenic plants. In Vitro Cell.Dev.Biol.-Plant 42, 601–606 (2006). https://doi.org/10.1079/IVP2006828
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2006828