Summary
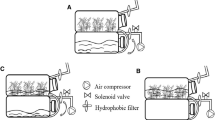
Plantlets propagated in temporary immersion bioreactors (TIB) have better performance than those propagated by conventional methods such as micropropagation. This is as a result of a better handling of the in vitro atmosphere and the nutrition. The object of this study to further improve the cultivation conditions by introducting photomixotrophism as an intermediate link of photoautotrophic growth during ex vitro acclimatization. For this purpose the effects of light were evaluated by different parameters such as photosynthetic photon flux density (PPF), sucrose concentration, and CO2 enrichment levels on CO2 evolution inside the culture vessels. It was observed that CO2 diminished upon light exposure and increased in the dark according to the photoperiod during each cycle of immersion. With this approach it was possible to increase the photomixotrophism in the pineapple plantlets propagated in TIB. It was demonstrated that light is the factor with more influence on plant quality, although under these conditions they seem to use more of the nutrients of the medium than their photoassimilates. The propagation of pineapple in TIB involves three phases: proliferation, pre-elongation, and final growth of the buds. In each phase the cultivation conditions were determined to substitute for sterilization by autoclaving, to improve the quality of the plants, to elevate the efficiency of the process, and to reduce production costs. The buds that grew in the temporary immersion bioreactor with the presence of Vitrofural (G-1) achieved the best indicators of growth. Significant increases were observed in the leaf area, dry mass of the buds, and chlorophyll contents.
Similar content being viewed by others
References
Cote, F.; Andre, M.; Folliot, M.; Massimino, D.; Daguenet, A. CO2 and O2 exchanges in the CAM plant Ananas comosus (L.) Merr. Determination of total and malate-decarboxylation-dependent CO2 assimilation rates. Study of light O2 uptake. Plant Physiol. 89:61–68; 1989.
Daquinta, M.; Benega, R. Brief review of tissue culture of pineapple. Pineapple Newsl. 3:7; 1997.
Desjardins, Y.; Gosselin, A.; Lamarre, M. Growth of transplants and in vitro culture clones of asparagus in response to CO2 enrichment and supplemental lighting. J. Am. Soc. Hort. Sci. 115:520–528;1990.
Escalona, M.; Lorenzo, J. C.; González, B.; Daquinta, M.; González, J.; Borroto, C.; Desjardins, Y. Pineapple (Ananas comosus L. Merr) plant propagation in temporary immersion system. Plant Cell Rep. 18:743–748; 1999.
Escalona, M.; Samson, G.; Borroto, C.; Desjardins, Y. Physiology of effects of temporary immersion bioreactors on micropropagated pineapple plantlets. In Vitro Cell. Dev. Biol. Plant 39:651–656; 2003.
Falkiner, F. R. The criteria for choosing an antibiotic for control of bacteria in plant tissue culture. Int. Soc. Plant Tiss. Cult. Newsl. 60:13–23; 1990.
Firoozabady, E.; Gutterson, N. Cost-effective in vitro propagation methods for pineapple. Plant Cell Rep. 21:844–850; 2003.
Firoozabady, E.; Nicholas, J.; Gutterson, N. In vitro plant regeneration and advanced propagation methods for pineapple. In Vitro (Abst.) 31:51A; 1995.
Heo, J.; Kozai, T. Forced ventilation micropropagation system for enhancing photosynthesis, growth and development of sweet potato plantlets. Environ. Control. Biol. 37(1):83–92; 1999.
Kadlecek, P.; Ticha, I.; Haisel, D.; Capkova, V.; Schaffer, C. H. Importance of in vitro pretreatment for ex vitro acclimatization and growth. Plant Sci. 161:695–701; 2001.
Kiyata, Y.; Mohapatra, S. C.; Kubota, C.; Kozai, T. Advantages of photoautotrophic micropropagation for space agriculture. In: Suge, H., ed. Plants in space biology. Proc. of workshop on several aspects of plant growth and development in space. November 17–18, 1995. Sendai, Japan: Tohoku University; 1996:235–244.
Laforge F.; Desjardins Y.; Lussier, C.; Gosselin, A. Effect of high photosynthetic flux density carbon dioxide enrichment during the in vitro rooting stage on the growth of strawberry, raspberry and asparagus plantlets in acclimatization. Sci. Hort. 47:259–269; 1991.
Le, V. Q.; Samson, G.; Desjardins, Y. Opposite effects of endogenous sucrose on growth, photosynthesis and carbon metabolism of in vitro plantlets of tomato grown under two levels of irradiance and CO2 concentration. J. Plant Physiol. 158:599–605;2001.
Leifert, C.; Waites, B.; Keetley, J. W.; Wright, S. M.; Nicholas, J. R.; Waites, W. M. Effect of medium acidification on filamentous fungi, yeast and bacterial contaminants in Delphinium tissue cultures. Plant Cell Tiss. Organ Cult. 36:149–155;1994.
Louche-Tessandier, D.; Samson, G.; Hernández-Sebastia, C.; Chagvardieff, P.; Desjardins, Y. Importance of light and CO2 on the effects of endomycorrhizal colonization on growth and photosynthesis of potato plantlets in an in vitro tripartite system. New Phytol. 149:539–550; 1999.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–479; 1962.
Nguyen, Q. T.; Kozai, T. Enviromental effects of the growth of plantlets in micropropagation. Environ. Control. Biol. 36(2):59–75 1998.
Niu, G.; Kozai, T.; Hayashi, M.; Tateno, M. Time course simulations of CO2 concentration and net photosynthetic rates of potato plantlets cultured under different lighting cycles. Trans. ASAE 40:1711–1718; 1998.
Porras, R. J. Recent advances and re-assessments in chlorophyll extraction and assay procedures for terrestrial, aquatic and marine organisms including recalcitrant algae. In: Scheer, H., ed. Chemistry of chlorophyll. Boca Raton, FL: CRC Press; 1991.
Winter, K. Crassulacean acid metabolism. In: Baker, J.; Baker, N. R., eds. Photosynthetis mechanisms and the environment. Amsterdam: Elsevier; 1985:329–387.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Olmedo, J.L., Fundora, Z., Molina, L.A. et al. New contributions to propagation of pineapple (Ananas comosus L. Merr) in temporary immersion bioreactors. In Vitro Cell.Dev.Biol.-Plant 41, 87–90 (2005). https://doi.org/10.1079/IVP2004603
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2004603