Abstract
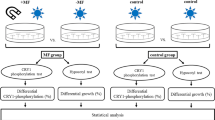
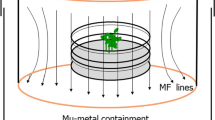
Cryptochromes are highly conserved blue light-absorbing flavoproteins which function as photoreceptors during plant development and in the entrainment of the circadian clock in animals. They have been linked to perception of electromagnetic fields in many organisms including plants, flies, and humans. The mechanism of magnetic field perception by cryptochromes is suggested to occur by the so-called radical pair mechanism, whereby the electron spins of radical pairs formed in the course of cryptochrome activation can be manipulated by external magnetic fields. However, the identity of the magnetosensitive step and of the magnetically sensitive radical pairs remains a matter of debate. Here we investigate the effect of a static magnetic field of 500 μT (10× earth’s magnetic field) which was applied in the course of a series of iterated 5 min blue light/10 min dark pulses. Under the identical pulsed light conditions, crypto-chrome responses were enhanced by a magnetic field even when exposure was provided exclusively in the 10 min dark intervals. However, when the magnetic stimulus was given exclusively during the 5 min light interval, no magnetic sensitivity could be detected. This result eliminates the possibility that magnetic field sensitivity could occur during forward electron transfer to the flavin in the course of the crypto-chrome photocycle. By contrast, radical pair formation during cryptochrome flavin reoxidation would occur independently of light, and continue for minutes after the cessation of illumination. Our results therefore provide evidence that a magnetically sensitive reaction is entwined with dark-state processes following the cryptochrome photoreduction step.
Similar content being viewed by others
Notes and references
I. Chaves, R. Pokorny, M. Byrdin, N. Hoang, T. Ritz, K. Brettel, L. O. Essen and G. T. van der Horst, Batschauer A, Ahmad M, The cryptochromes: blue light photoreceptors in plants and animals, Annu. Rev. Plant Biol., 2011, 62, 335–364, DOI: 10.1146/annurev-arplant-042110–103759.
N. Ozturk, Phylogenetic and Functional Classification of the Photolyase/Cryptochrome Family, Photochem. Photobiol., 2017, 93(1), 104–111, DOI: 10.1111/php.12676.
Z. Yang, B. Liu, J. Su, J. Liao, C. Lin and Y. Oka, Cryptochromes Orchestrate Transcription Regulation of Diverse Blue Light Responses in Plants, Photochem. Photobiol., 2017, 93(1), 112–127, DOI: 10.1111/php.l2663.
X. Wang, Q. Wang, P. Nguyen and C. Lin, Cryptochrome-mediated light responses in plants, Enzymes, 2014, 35, 167–189, DOI: 10.1016/B978-0-12-801922-1.00007-5.
V. D’Amico-Damiao and R. F. Carvalho, Cryptochrome-Related Abiotic Stress Responses in Plants, Front. Plant Sci., 2018, 9, 1897, DOI: 10.3389/fpls.2018.01897. eCollection 2018.
Q. Wang, Z. Zuo, X. Wang, Q. Liu, L. Gu, Y. Oka and C. Lin, Beyond the photocycle-how cryptochromes regulate photo-responses in plants?, Curr. Opin. Plant Biol., 2018, 45(Pt A), 120–126, DOI: 10.1016/j.pbi.2018.05.014, Epub 2018 Jun 15.
T. Kleine, P. Lockhart and A. Batschauer, An Arabidopsis protein closely related to Synechocystis cryptochrome is targeted to organelles, Plant J., 2003, 35, 93–103.
A. Hense, E. Herman, S. Oldemeyer and T. Kottke, Proton transfer to flavin stabilizes the signaling state of the blue light receptor plant cryptochrome, J. Biol. Chem., 2015, 290, 1743–1751.
P. Muller and M. Ahmad, Light activated cryptochrome reacts with molecular oxygen to form a flavin-superoxide radical pair consistent with magnetoreception, J. Biol. Chem., 2011, 286, 21033–21040.
J. P. Bouly, E. Schleicher, M. Dionisio-Sese, F. Vandenbussche, D. Van Der Straeten, N. Bakrim, S. Meier, A. Batschauer, P. Galland, R. Bittl and M. Ahmad, Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states, J. Biol. Chem., 2007, 282, 9383–9391.
M. Procopio, J. Link, D. Engle, J. Witczak, T. Ritz and M. Ahmad, Kinetic Modeling of the Arabidopsis Cryptochrome Photocycle: FADH(o) Accumulation Correlates with Biological Activity, Front. Plant Sci., 2016, 7, 888, DOI: 10.3389/fpls.2016.00888, eCollection 2016.
C. Lin, D. Top, C. C. Manahan, M. W. Young and B. R. Crane, Circadian clock activity of cryptochrome relies on tryptophan-mediated photoreduction, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(15), 3822–3827, DOI: 10.1073/ pnas.1719376115, First published March 26, 2018.
M. Ahmad, Photocycle and signaling mechanisms of plant cryptochromes, Curr. Opin. Plant Biol., 2016, 33, 108–115, DOI: 10.1016/j.pbi.2016.06.013, Epub 2016 Jul 14.
V. Herbel, C. Orth, R. Wenzel, M. Ahmad, R. Bittl and A. Batschauer, Lifetimes of Arabidopsis cryptochrome signaling states in vivo, Plant J., 2013, 74, 583–592.
R. Banerjee, E. Schleicher, S. Meier, R. M. Viana, R. Pokorny, M. Ahmad, R. BittI and A. J. Batschauer, The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone, Biol. Chem., 2007, 282, 14916–14922.
M. Kondoh and M. Terazima, Conformational and Intermolecular Interaction Dynamics of Photolyase/ Cryptochrome Proteins Monitored by the Time-Resolved Diffusion Technique, Photochem. Photobiol., 2017, 93(1), 15–25, DOI: 10.1111/php.12681, Epub 2017 Jan 9. Review.
M. Kondoh, C. Shiraishi, P. Muller, M. Ahmad, K. Hitomi, E. D. Getzoff and M. Terazima, Light-induced conformational changes in full-length Arabidopsis thaliana cryptochrome, J. Mol. Biol., 2011, 413, 128–137.
C. Thöing, S. Oldemeyer and T. Kottke, Microsecond Deprotonation of Aspartic Acid and Response of the α/β Subdomain Precede C-Terminal Signaling in the Blue Light Sensor Plant Cryptochrome, J. Am. Chem. Soc, 2015, 137(18), 5990–5999, DOI: 10.1021/jacs.5b01404, Epub 2015 May 4.
A. Zeugner, M. Byrdin, J. P. Bouly, N. Bakrim, B. Giovani, K. Brettel and M. Ahmad, Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function, J. Biol. Chem., 2005, 280, 19437–19440.
X. Li, Q. Wang, X. Yu, H. Liu, H. Yang, C. Zhao, X. Liu, C. Tan, J. Klejnot, D. Zhong and C. Lin, Arabidopsis cryptochrome 2 (CRY2) functions by the photoactivation mechanism distinct from the tryptophan (trp) triad-dependent photoreduction, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20844–20849.
J. Gao, X. Wang, M. Zhang, M. Bian, W. Deng, Z. Zuo, Z. Yang, D. Zhong and C. Lin, Trp triad-dependent rapid photoreduction is not required for the function of Arabidopsis CRY1, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 9135–9140.
Z. Yang, B. Liu, J. Su, J. Liao, C. Lin and Y. Oka, Cryptochromes Orchestrate Transcription Regulation of Diverse Blue Light Responses in Plants, Photochem. Photobiol., 2017, 93(1), 112–127, DOI: 10.1111/php.12663, Epub 2017 Jan 27.
I. H. Kavakli, I. Baris, M. Tardu, Ş. Gül, H. Öner, S. Çal, S. Bulut, D. Yarparvar, Ç. Berkel, P. Ustaoğlu and C. Aydin, The Photolyase/Cryptochrome Family of Proteins as DNA Repair Enzymes and Transcriptional Repressors, Photochem. Photobiol., 2017, 93(1), 93–103, DOI: 10.1111/ php.12669, Epub 2017 Jan 9. Review.
M. El-Esawi, L. D. Arthaut, N. Jourdan, A. d’Harlingue, J. Link, C. F. Martino and M. Ahmad, Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome, Sci Rep., 2017, 7(1), 13875, DOI: 10.1038/s41598-017-13832-z.
L. Consentino, S. Lambert, C. Martino, N. Jourdan, P.-E. Bouchet, J. Witczak, P. Castello, M. El-Esawi, F. Corbineau, A. D’Harlingue and M. Ahmad, Blue-light dependent ROS formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism, New Phytol., 2015, 206(4), 1450–1462, DOI: 10.1111/ nph.13341, Epub 2015 Feb 26, New Phytologist ROS.
Q. Wang, D. Barshop William, M. Bian, A. Vashisht Ajay, R. He, X. Yu, B. Liu, P. Nguyen, X. Liu, X. Zhao, et al., The blue light-dependent phosphorylation of the CCE domain determines the photosensitivity of Arabidopsis CRY2, Mol. Plant, 2015, 8, 631–643. [PubMed: 25792146].
X. Wang, Q. Wang, Y.-J. Han, Q. Liu, L. Gu, Z. Yang, J. Su, B. Liu, Z. Zuo, W. He, et al., A CRY-BIC negative feedback circuitry regulating blue light sensitivity of Arabidopsis, Plant J., 2017, 92, 426–436. [PubMed: 28833729].
C. Nießner, S. Denzau, K. Stapput, M. Ahmad, L. Peichl, W. Wiltschko and R. Wiltschko, Magnetoreception: activated cryptochrome la concurs with magnetic orientation in birds, J. R. Soc, Interface, 2013, 10(88), 20130638, DOI: 10.1098/rsif.2013.0638.
R. Wiltschko and W. Wiltschko, Sensing magnetic directions in birds: radical pair processes involving cryptochrome, Biosensors, 2014, 4(3), 221–242, DOI: 10.3390/ bios4030221.
C. Xu, X. Yin, Y. Lv, C. Wu, Y. Zhang and T. Song, A near-null magnetic field affects cryptochrome- related hypocotyl growth and flowering in Arabidopsis, Adv. Space Res., 2012, 49(5), 834–840, DOI: 10.1016/j.asr.2011.12.004.
C. Xu, Y. Lv, C. Chen, Y. Zhang and S. Wei, Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis, Adv. Space Res., 2014, 53(7), 1118–1124.
C. Xu, Y. Li, Y. Yu, Y. Zhang and S. Wei, Suppression of Arabidopsis flowering by near-null magnetic field is affected by light, Bioelectromagnetics, 2015, 36(6), 476–479, DOI: 10.1002/bem.21927.
M. Pooam, L. D. Arthaut, D. Burdick, J. Link, C. F. Martino and M. Ahmad, Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark, Planta, 2019, 249(2), 319–332, DOI: 10.1007/s00425–018–3002–y.
C. L. Wu, T. F. Fu, M. H. Chiang, Y. W. Chang, J. L. Her, et al., Magnetoreception regulates male courtship activity in Drosophila, PLoS One, 2016, 11(5), e0155942, DOI: 10.1371/journal.pone.0155942.
T. Yoshii, M. Ahmad and C. Helfrich-Forster, Cryptochrome mediates light-dependent magnetosensitiv-ity of Drosophila’s circadian clock, PLoS Biol., 2009, 7(4), e1000086, DOI: 10.1371/journal.pbio.1000086.
G. Fedele, E. W. Green, E. Rosato and C. P. Kyriacou, An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway, Nat. Commun., 2014, 5, 4391, DOI: 10.1038/ncomms5391.
P. J. Hore and H. Mouritsen, The Radical-Pair Mechanism of Magnetoreception, Annu. Rev. Biophys., 2016, 45, 299–344, DOI: 10.1146/annurev-biophys-032116–094545.
E. W. Evans, C. A. Dodson, K. Maeda, T. Biskup, C. J. Wedge and C. R. Timmel, Magnetic field effects in fla-voproteins and related systems, Interface Focus, 2013, 3(5), 20130037, DOI: 10.1098/rsfs.2013.0037.
R. Wiltschko, M. Ahmad, C. Nießner, D. Gehring and W. Wiltschko, Light-dependent magnetoreception in birds: the crucial step occurs in the dark. Light-dependent magnetoreception in birds: the crucial step occurs in the dark, J. R. Soc, Interface, 2016, 13, 20151010, DOI: 10.1098/ rsif.2015.1010.
Z. Zeng, J. Wei, Y. Liu, W. Zhang and T. Mabe, Magnetoreception of Photoactivated Cryptochrome 1 in Electrochemistry and Electron Transfer, ACS Omega, 2018, 3, 4752–4759, DOI: 10.1021/acsomega.8b00645.
T. Ritz, et al., Magnetic Compass of Birds Is Based on a Molecule with Optimal Directional Sensitivity, Biophys. J., 2009, 96(8), 3451–3457.
H. J. Hogben, P. J. Hore and I. Kuprov, Strategies for state space restriction in densely coupled spin systems with applications to spin chemistry, J. Chem. Phys., 2010, 132, 174101, DOI: 10.1063/1.3398146.
A. A. Lee, J. C. S. Lau, H. H. Hogben, T. Biskup, D. R. Kattnig and P. J. Hore, Alternative radical pairs for cryptochrome-based magnetoreception, J. R. Soc, Interface, 2014, DOI: 10.1098/rsif.2013.1063.
P. Mondal and M. Huix-Rotllant, Theoretical insights into the formation and stability of radical oxygen species in cryptochromes, Phys. Chem. Chem. Phys., 2019, 21, 8874–8882, DOI: 10.1039/c9cp00782b.
D. Kattnig, Radical-Pair-Based Magnetoreception Amplified by Radical Scavenging: Resilience to Spin Relaxation, J. Phys. Chem. B, 2017, 121(44), 10215–10227.
D. M. W. Sheppard, J. Li, K. B. Henbest, S. R. T. Neil, K. Maeda, J. Storey, E. Schleicher, T. Biskup, R. Rodríguez, S. Weber, P. J. Hore, C. R. Timmel and S. R. Mackenzie, Millitesla magnetic field effects on the photocycle of an animal cryptochrome, Sci Rep., 2017, 7, 42228.
D. Nohr, B. Paulus, R. Rodríguez, et al., Determination of Radical-Radical Distances in Light Active Proteins and Their Implication for Biological Magnetoreception, Angew. Chem., Int. Ed., 2017, 56, 8550–8554, DOI: 10.1002/ anie.201700389.
B. D. Zoltowski, Y. Chelliah, A. Wickramaratne, L. Jarocha, N. Karki, W. Xu, H. Mouritsen, P. J. Hore, R. E. Hibbs, C. B. Green and J. S. Takahashi, Chemical and structural analysis of a photoactive vertebrate cryptochrome from pigeon, Proc Natl. Acad. Sci U. S. A., 2019, 116(39), 19449–19457, DOI: 10.1073/pnas.1907875116.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hammad, M., Albaqami, M., Pooam, M. et al. Cryptochrome mediated magnetic sensitivity in Arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem Photobiol Sci 19, 341–352 (2020). https://doi.org/10.1039/c9pp00469f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00469f