Effect of GCAA stabilizing loops on three- and four-way intramolecular junctions†
Abstract
Tetraloops are a common way of changing the melting behavior of a DNA or RNA structure without changing the sequence of the stem. Because of the ubiquitous nature of tetraloops, our goal is to understand the effect a GCAA tetraloop, which belongs to the GNRA family of tetraloops, has on the unfolding thermodynamics of intramolecular junctions. Specifically, we have described the melting behavior of intramolecular three-way and four-way junctions where a T5 loop has been replaced with a GCAA tetraloops in different positions. Their thermodynamic profiles, including ΔnNa+ and ΔnW, were analyzed based on the position of the tetraloop. We obtained 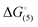 between −16.7 and −27.5 kcal mol−1 for all junctions studied. The experimental data indicates the influence of the GCAA tetraloop is primarily dictated by the native unfolding of the junction; if the tetraloop is placed on a stem that unfolds as a single domain when the tetraloop is not present, it will unfold as a single domain when the tetraloop is present but with a higher thermal stability. Conversely, if the tetraloop is placed on a stem which unfolds cooperatively with other stems when the tetraloop is not present, the tetraloop will increase the thermal stability of all the stems in the melting domain. The oligonucleotide structure and not the tetraloop itself affects ion uptake; three-way junctions do not gain an increase in ion uptake, but four-way junctions do. This is not the case for water immobilization, where the position of the tetraloop dictates the amount of water immobilized.
between −16.7 and −27.5 kcal mol−1 for all junctions studied. The experimental data indicates the influence of the GCAA tetraloop is primarily dictated by the native unfolding of the junction; if the tetraloop is placed on a stem that unfolds as a single domain when the tetraloop is not present, it will unfold as a single domain when the tetraloop is present but with a higher thermal stability. Conversely, if the tetraloop is placed on a stem which unfolds cooperatively with other stems when the tetraloop is not present, the tetraloop will increase the thermal stability of all the stems in the melting domain. The oligonucleotide structure and not the tetraloop itself affects ion uptake; three-way junctions do not gain an increase in ion uptake, but four-way junctions do. This is not the case for water immobilization, where the position of the tetraloop dictates the amount of water immobilized.



 Please wait while we load your content...
Please wait while we load your content...