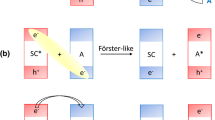
Abstract
This study investigates the effect of reaction temperature (298-353 K) on photocatalytic H2 production in bare and platinized TiO2 (Pt/TiO2) suspensions containing various organic hole scavengers (EDTA, methanol, and formic acid) under UV (λ > 320 nm) and visible light (λ > 420 nm for ligand-to-metal charge transfer). H2 production rates are enhanced ~7.8- and ~2.5-fold in TiO2 and Pt/TiO2 suspensions, respectively, with EDTA under UV by simply elevating the reaction temperature from 298 K to 323 K (ΔT = 25 °C). Such a temperature-boosted increase in H2 production is always observed, regardless of the TiO2 crystalline structure (anatase, rutile, and an anatase/rutile mixture), type of hole scavenger, and irradiation wavelength range. It is estimated that approximately 90% of incident photons are utilized in H2 production, for which the activation energy is 25.5 kJ mol−1. Detailed photoelectrochemical analyses show the positive relationship between reaction temperature and photocurrent generation, with charge carrier mobility and interfacial charge transfer improving at higher temperatures. Other possible factors, such as H2 solubility and mass transport, play a limited role.
Similar content being viewed by others
References
Semiconductor Electrodes, ed. H. O. Finklea, Elsevier, Amsterdam, 1988.
N. Serpone and E. Pelizzetti, Photocatalysis: Fundamentals and Applications, Wiley, New York, 1989.
R. Van de Krol and M. Gratzel, Photoelectrochemical Hydrogen Production, Springer, New York, 2012.
On Solar Hydrogen & Nanotechnology, ed. L. Vayssieres, Wiley, Singapore, 2009.
K. Maeda and K. Domen, Photocatalytic Water Splitting: Recent Progress and Future Challenges, J. Phys. Chem. Lett., 2010, 1, 2655–2661.
J. S. Jang and H. Park, in Materials and Processes for Solar Fuel Production, ed. R. Subramanian, B. Viswanathan and J. S. Lee, Springer, 2014.
D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Photocatalyst releasing hydrogen from water, Nature, 2006, 440, 295.
H. Kato, M. Hori, R. Konta, Y. Shimodaira and A. Kudo, Construction of Z-scheme type heterogeneous photocatalysis systems for water splitting into H2 and O2 under visible light irradiation, Chem. Lett., 2004, 33, 1348–1349.
T. H. Jeon, W. Choi and H. Park, Cobalt-phosphate complexes catalyze the photoelectrochemical water oxidation of BiVO4 electrodes, Phys. Chem. Chem. Phys., 2011, 13, 21392–21401.
S. K. Choi, W. Choi and H. Park, Solar water oxidation using nickel-borate coupled BiVO4 photoelectrodes, Phys. Chem. Chem. Phys., 2013, 15, 6499–6507.
H. W. Jeong, T. H. Jeon, J. S. Jang, W. Choi and H. Park, Strategic modification of BiVO4 for improving photoelectrochemical water oxidation performance, J. Phys. Chem. C, 2013, 117, 9104–9112.
H. W. Jeong, S.-W. Chae, B. Song, C.-H. Cho, S.-H. Baek, Y. Park and H. Park, Optical resonance and charge transfer behavior on patterned WO3 microdisc arrays, Energy Environ. Sci., 2016 DOI: 10.1039/C1036EE01003B.
H. Park, H.-H. Ou, U. Kang, J. Choi and M. R. Hoffmann, Photocatalytic conversion of carbon dioxide to methane on TiO2/CdS in aqueous isopropanol solution, Catal. Today, 2016, 266, 153–159.
H. Park, W. Choi and M. R. Hoffmann, Effects of the preparation method of the ternary CdS/TiO2/Pt hybrid photocatalysts on visible light-induced hydrogen production, J. Mater. Chem., 2008, 18, 2379–2385.
A. Mills and R. Davies, Activation energies in semiconductor photocatalysis for water purification: the 4-chlorophenol-TiO2-O2 photosystem, J. Photochem. Photobiol., A, 1995, 85, 173–178.
J.-F. Wu, C.-H. Hung, C.-S. Yuan, Kinetic modeling of promotion and inhibition of temperature on photocatalytic degradation of benzene vapor, J. Photochem. Photobiol., A, 2005, 170, 299–306.
X. Fu, L. A. Clark, W. A. Zeltner and M. A. Anderson, Effects of reaction temperature and water vapor content on the heterogeneous photocatalytic oxidation of ethylene, J. Photochem. Photobiol., A, 1996, 97, 181–186.
F. Saladin and I. Alxneit, Temperature dependence of the photochemical reduction of CO2 in the presence of H2O at the solid/gas interface of TiO2, J. Chem. Soc., Faraday Trans., 1997, 93, 4159–4163.
G. Kim and W. Choi, Charge-transfer surface complex of EDTA-TiO2 and its effect on photocatalysis under visible light, Appl. Catal., B, 2010, 100, 77–83.
H. Park, in Engineered Nanoparticles and the Environment: Biophysicochemical Processes and Toxicity, ed. B. Xing, C. D. Vecitis and N. Senesi, Wiley, New York, 2016.
H. Park, Y. Park, W. Kim and W. Choi, Surface modification of TiO2 photocatalyst for environmental applications, J. Photochem. Photobiol., C, 2013, 15, 1–20.
H. Park, H.-i. Kim, G.-h. Moon and W. Choi, Photoinduced charge transfer processes in solar photocatalysis based on modified TiO2, Energy Environ. Sci., 2016, 9, 411–433.
J. Ryu and W. Choi, Substrate-specific photocatalytic activities of TiO2 and multiactivity test for water treatment application, Environ. Sci. Technol., 2008, 42, 294–300.
H. Park and W. Choi, Photoelectrochemical investigation on electron transfer mediating behaviors of polyoxometalate in UV-illuminated suspensions of TiO2 and Pt/TiO2, J. Phys. Chem. B, 2003, 107, 3885–3890.
H. Park, A. Bak, T. H. Jeon, S. Kim and W. Choi, Photo-chargeable and dischargeable TiO2 and WO3 heterojunction electrodes, Appl. Catal., B, 2012, 115, 74–80.
S. Kim and H. Park, Sunlight-harnessing and storing heterojunction TiO2/Al2O3/WO3 electrodes for night-time applications, RSC Adv., 2013, 3, 17551–17558.
S. Kim, Y. Park, W. Kim and H. Park, Harnessing and storing visible light using a heterojunction of WO3 and CdS for sunlight-free catalysis, Photochem. Photobiol. Sci., 2016, 15, 1006–1011.
H. Park, J. Lee and W. Choi, Study of special cases where the enhanced photocatalytic activities of Pt/TiO2 vanish under low light intensity, Catal. Today, 2006, 111, 259–265.
W. Kim, T. Tachikawa, G. Moon, T. Majima and W. Choi, Molecular-level understanding of the photocatalytic activity difference between anatase and rutile nanoparticles, Angew. Chem., Int. Ed., 2014, 53, 14036–14041.
A. F. Alkaim, T. A. Kandiel, F. H. Hussein, R. Dillert and D. W. Bahnemann, Enhancing the photocatalytic activity of TiO2 by pH control: A case study for the degradation of EDTA, Catal. Sci. Technol., 2013, 3, 3216–3222.
L. M. Ahmed, F. H. Hussein and A. A. Mahdi, Photocatalytic dehydrogenation of aqueous methanol solution by naked and platinized TiO2 nanoparticles, Asian J. Chem., 2012, 24, 5564–5568.
Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press, New York, 90th edn, 2009.
Y.-K. Hsu, Y.-C. Chen, Y.-G. Lin, L.-C. Chen, K.-H. Chen, Birnessite-type manganese oxides nanosheets with hole acceptor assisted photoelectrochemical activity in response to visible light, J. Mater. Chem., 2012, 22, 2733–2739.
D. K. Zhong and D. R. Gamelin, Photoelectrochemical water oxidation by cobalt catalyst/α-Fe2O3 composite photoanodes: Oxygen evolution and resolution of a kinetic bottleneck, J. Am. Chem. Soc., 2010, 132, 4202–4207.
T. Hisatomi, K. Maeda, K. Takanabe, J. Kubota and K. Domen, Aspects of the water splitting mechanism on (Ga1−xZnx)(N1−xOx) photocatalyst modified with Rh2−yCryO3 cocatalyst, J. Phys. Chem. C, 2009, 113, 21458–21466.
S. A. Sherif, D. Y. Goswami, E. K. Stefanakos and A. Steinfeld, Handbook of Hydrogen Energy, CRC Press, New York, 2014.
N. Kopidakis, K. D. Benkstein, J. van de Lagemaat and A. J. Frank, Temperature dependence of the electron diffusion coefficient in electrolyte-filled TiO2 nanoparticle films: Evidence against multiple trapping in exponential conduction-band tails, Phys. Rev. B: Condens. Matter, 2006, 73, 045326.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available: Fig. S1-S4. See DOI: 10.1039/c6pp00263c
Rights and permissions
About this article
Cite this article
Kim, G., Choi, H.J., Kim, Hi. et al. Temperature-boosted photocatalytic H2 production and charge transfer kinetics on TiO2 under UV and visible light. Photochem Photobiol Sci 15, 1247–1253 (2016). https://doi.org/10.1039/c6pp00263c
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c6pp00263c