Abstract
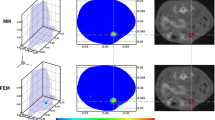
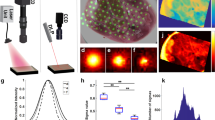
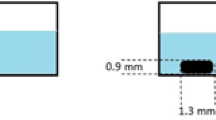
Understanding the interactions of non-ionizing radiation with living organisms has been the focus of much research over recent decades. The complex nature of these interactions warrants development of theoretical and experimental studies to gain an insight into predicting and monitoring the success of photodynamic therapy (PDT) protocols. There is a major impetus towards evidence-based recommendations for patient diagnosis, treatment and management. Knowledge of the biophysical aspects of PDT is important for improving dosimetry protocols. Fluorescence in clinical PDT may be used to detect and diagnose pre-malignant and malignant conditions, while photobleaching can monitor changes in fluorescence during treatment. Combining empirical fluorescence photobleaching clinical data with computational modelling enables clinical PDT dosimetry protocols to be investigated with a view to optimising treatment regimes. We will discuss how Monte Carlo radiation transfer (MCRT) modelling has been intercalated in the field of fluorescence detection and PDT. In this paper we highlight important aspects of basic research in PDT by reporting on the current utilisation of fluorescence in clinical PDT from both a clinical and theoretical perspective. Understanding and knowledge of light propagation in biological tissue from these perspectives should have a positive impact on treatment planning.
Similar content being viewed by others
References
L. R. Braathen, R.-M. Szeimies, N. Basset-Seguin, R. Bissonnette, P. Foley, D. Pariser, R. Roelandts, A.-M. Wennberg, and C. A. Morton, Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus, J. Am. Acad. Dermatol., 2007, 56, 125–143.
C. A. Morton, K. E. McKenna, and L. E. Rhodes, Guidelines for topical photodynamic therapy: update, Br. J. Dermatol., 2008, 159, 1245–1266.
S. Nonell, and R. W. Redmond, On the determination of quantum yields for singlet molecular oxygen photosensitization, J. Photochem. Photobiol., B., 1994, 22, 171–172.
O. Raab, Uber die Wirkung fluoreszierender Stoffe auf Infusorien, Z. Biol., 1900, 39, 524–546.
R. L. Lipson, and E. J. Baldes, The photodynamic properties of a particular hematoporphyrin derivative, Arch. Dermatol., 1960, 82, 508–516.
R. L. Lipson, E. J. Baldes, and A. M. Olsen, The use of a derivative of hematoporphyrin in tumor detection, J. Natl. Cancer Inst., 1961, 26, 1–11.
T. J. Dougherty, Photoradiation therapy for the treatment of malignant tumours, Cancer Res., 1978, 36, 2628–2635.
J. C. Kennedy, R. H. Pottier, and D. C. Pross, Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience, J. Photochem. Photobiol., B, 1990, 6, 143–148.
A. Policard, Etudes sur les aspects offerts par des tumeurs experimentales examinees a la lumiere de Woods, C. R. Soc. Biol., 1924, 91, 1423–1425.
A. Lesar, J. Ferguson, and H. Moseley, A time course investigation of the fluorescence induced by topical application of 5-aminolevulinic acid and methyl aminolevulinate on normal human skin, Photodiagn. Photodyn. Ther., 2011, 8(2), 97–103.
A. Lesar, J. Ferguson, and H. Moseley, An investigation of the fluorescence induced by topical application of 5-aminolevulinic acid and methyl aminolaevulinate at different body sites on normal human skin, Photodermatol. Photoimmunol. Photomed., 2009, 25(4), 191–195.
S. H. Ibbotson, C. Jong, A. Lesar, J. S. Ferguson, M. Padgett, M. O‘Dwyer, R. Barnetson, and J. Ferguson, Characteristics of 5-aminolaevulinic acid-induced protoporphyrin IX fluorescence in human skin in vivo, Photodermatol. Photoimmunol. Photomed., 2006, 22(2), 105–110.
N. van der Beek, J. de Leeuw, C. Demmendal, P. Bjerring, H. A. M. Neumann, PpIX fluorescence combined with auto-fluorescence is more accurate than PpIX fluorescence alone in fluorescence detection of non-melanoma skin cancer: an intra-patient direct comparison study, Lasers Surg. Med., 2012, 44, 271–276.
J. Q. Brown, K. Vishwanath, G. M. Palmer, and N. Ramanujam, Advances in quantitative UV-visible spectroscopy for clinical and pre-clinical application in cancer, Curr. Opin. Biotechnol., 2009, 20, 119–131.
N. Rajaram, T. J. Aramil, K. Lee, J. S. Reichenberg, T. H. Nguyen, and J. W. Tunnell, Design and validation of a clinical instrument for spectral diagnosis of cutaneous malignancy, Appl. Opt., 2010, 49(2), 142–152.
B. C. Wilson, M. S. Patterson, and L. Lilge, Implicit and explicit dosimetry in photodynamic therapy: a new paradigm, Lasers Med. Sci., 1997, 12, 182–199.
T. J. Farrell, R. P. Hawkes, M. S. Patterson, and B. C. Wilson, Modeling of photosensitizer fluorescence emission and photobleaching for photodynamic therapy dosimetry, Appl. Opt., 1998, 37(31), 7168–7183.
A. J. L. Jongen, H. J. C. M. Sterenborg, Mathematical description of photobleaching in vivo describing the influence of tissue optics on measured fluorescence signals, Phys. Med. Biol., 1997, 42, 1701–1716.
R. M. Valentine, C. T. A. Brown, H. Moseley, S. Ibbotson, and K. Wood, Monte Carlo modeling of in vivo protoporphyrin IX fluorescence and singlet oxygen production for patients presenting with superficial basal cell carcinoma, J. Biol. Opt., 2011, 16(4), 048002.
K. Badizadegan, V. Backman, C. W. Boone, C. P. Crum, R. R. Dasari, I. Georgakoudi, K. Keefe, K. Munger, S. M. Shapshay, E. E. Sheets, and M. S. Feld, Spectroscopic diagnosis and imaging of invisible pre-cancer, Faraday Discuss., 2004, 126, 265–279.
G. A. Wagnieres, W. M. Star, and B. C. Wilson, In vivo fluorescence spectroscopy and imaging for oncological applications, Photochem. Photobiol., 1998, 68(5), 603–632.
L. Coghlan, U. Utzinger, R. Richards-Kortum, C. Brookner, A. Zuluaga, I. Gimenez-Conti, and M. Follen, Fluorescence spectroscopy of epithelial tissue throughout the dysplasia-carcinoma sequence in an animal model: spectroscopic changes precede morphological changes, Lasers Surg. Med., 2001, 29, 1–10.
R. Richards-Kortum, E. Sevick-Muraca, Quantitative optical spectroscopy for tissue diagnosis, Annu. Rev. Phys. Chem., 1996, 47, 555–606.
N. Kollias, G. Zonios, and G. N. Stamatas, Fluorescence spectroscopy of skin, Vib. Spectrosc., 2002, 28(1), 17–23.
L. Brancaleon, A. J. Durkin, J. H. Tu, G. Menaker, J. D. Fallon, and N. Kollias, In vivo fluorescence spectroscopy of nonmelanoma skin cancer, Photochem. Photobiol., 2001, 73(2), 178–183.
R. F. V. Lopez, N. Lange, R. Guy, M. V. L. B. Bentley, Photodynamic therapy of skin cancer: controlled drug delivery of 5-ALA and its esters, Adv. Drug Delivery Rev., 2003, 56, 77–94.
L. O. Svaasand, B. J. Tromberg, P. Wyss, M.-T. Wyss-Desserich, Y. Tadir, and M. W. Berns, Light and drug distribution with topically administered photosensitizers, Lasers Med. Sci., 1996, 11(4), 261-165.
J. C. Finlay, L. Jun, X. Zhou, and T. C. Zhu, Patient-specific dosimetry for photodynamic therapy, Proc. SPIE-Int. Soc. Opt. Eng., 2008, 12III, 115–125.
J. Tyrrell, S. M. Campbell, and A. Curnow, Monitoring the accumulation and dissipation of the photosensitizer protoporphyrin IX during standard dermatological methyl-aminolevulinate photodynamic therapy utilizing non-invasive fluorescence imaging and quantification, Photodyn. Photodiagn. Ther., 2011, 8(1), 30–38.
Y. Won, S. H. Hong, H. Y. Yu, Y. H. Kwon, S. J. Yun, S. C. Lee, and J. B. Lee, Photodetection of basal cell carcinoma using methyl 5-aminolaevulinate-induced protoporphyrin IX based on fluorescence image analysis, Clin. Exp. Dermatol., 2007, 32(4), 423–429.
R. M. Valentine, S. H. Ibbotson, C. T. A. Brown, K. Wood, and H. Moseley, A quantitative comparison of 5-aminolaevulinic acid- and methyl aminolevulinate-induced fluorescence, photobleaching and pain during photodynamic therapy, Photochem. Photobiol., 2011, 87(1), 242–249.
D. J. Robinson, H. S. de Bruin, N. van der Veen, M. R. Stringer, S. B. Brown, and W. M. Star, Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: the effect of light dose and irradiance and the resulting biological effect, Photochem. Photobiol., 1998, 67(1), 140–149.
K. R. Weishaupt, C. J. Gomer, and T. J. Dougherty, Identification of singlet oxygen as cytotoxic agent in photo-inactivation of a murine tumor, Cancer Res., 1976, 36, 2326–2329.
M. S. Patterson, B. C. Wilson, and R. Graff, In vivo tests of the concept of photodynamic threshold dose in normal rat liver photosensitized by aluminium chlorosulphonated phthalocyanine, Photochem. Photobiol., 1990, 51, 343–349.
S. L. Jacques, R. Joseph, and G. Gofstein, How photobleaching affects dosimetry and fluorescence monitoring of PDT in turbid media, Proc. SPIE-Int. Soc. Opt. Eng., 1993, 1881, 168–179.
M. J. Niedre, C. S. Yu, M. S. Patterson, and B. C. Wilson, Singlet oxygen luminescence as an in vivo photodynamic therapy dose metric: validation in normal mouse skin with topical amino-levulinic acid, Br. J. Cancer, 2005, 92(2), 298–304.
T. C. Zhu, and J. C. Finlay, The role of photodynamic therapy (PDT) physics, Med. Phys., 2008, 35(7), 3127–3136.
B. W. Henderson, T. M. Busch, L. A. Vaughan, N. P. Frawley, D. Babich, T. A. Sosa, J. D. Zollo, A. S. Dee, M. T. Cooper, D. A. Bellnier, W. R. Greco, and A. R. Oseroff, Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate, Cancer Res., 2000, 60, 525–529.
J. Tyrrell, C. Thorn, A. Shore, S. Campbell, and A. Curnow, Oxygen saturation and perfusion changes during dermatological methylaminolaevulinate photodynamic therapy, Br. J. Dermatol., 2011, 165(6), 1323–1331.
W.-F. Cheong, S. A. Prahl, and A. J. Welch, A review of the optical properties of biological tissues, IEEE J. Quantum Electron., 1990, 26(12), 2166–2185.
L. V. Wang, and H.-I. Wu, Biomedical Optics: Principles and Imaging, John Wiley & Sons, Inc., 2007, 88.
S. Chandrasekhar, Radiative Transfer, Dover Publications, Inc., 1960, 1.
P. Prasad, Introduction to Biophotonics, John Wiley & Sons, Inc., 2003, 169.
L. Carroll, and T. R. Humphreys, Laser–tissue interactions, Clin. Dermatol., 2006, 24, 2–7.
J. Swartling, J. Svensson, D. Bengtsson, K. Terike, S. Andersson-Engels, Fluorescence spectra provide information on the depth of fluorescent lesions in tissue, Appl. Opt., 2005, 44(10), 1934–1941.
A. E. Profio, and D. R. Doiron, Transport of light in tissue in photodynamic therapy, Photochem. Photobiol., 1987, 46(5), 591–599.
J. L. Sandell, and T. C. Zhu, A review of in vivo optical properties of human tissues and its impact on PDT, J. Biophotonics, 2011, 4, 773–787.
W. J. Cottrell, A. D. Paquette, K. R. Keymel, T. H. Foster, and A. R. Oseroff, Irradiance-dependent photobleaching and pain in δ-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas, Clin. Cancer Res., 2008, 14(14), 4475–4483.
M. B. Ericson, C. Sandberg, B. Stenquist, F. Gudmundson, M. Karlsson, A.-M. Ros, A. Rosen, O. Larko, A.-M. Wennberg, and I. Rosdahl, Photodynamic therapy of actinic keratosis at varying fluence rates: assessment of photobleaching, pain and primary clinical outcome, Br. J. Dermatol., 2004, 151, 1204–1212.
B. C. Wilson, and M. S. Patterson, The physics, biophysics and technology of photodynamic therapy, Phys. Med. Biol., 2008, 53, R61–R109.
G. Yoon, S. A. Prahl, and A. J. Welch, Accuracies of the diffusion approximation and its similarity relations for laser irradiated biological media, Appl. Opt., 1989, 28(12), 2250–2255.
L. Wang, S. L. Jacques, and L. Zheng, MCML–Monte Carlo modeling of light transport in multi-layered tissues, Comput. Methods Prog. Biomed., 1995, 47, 131–146.
S. A. Prahl, M. Keijzer, S. L. Jacques, and A. J. Welch, A Monte Carlo model of light propagation in tissue, Proc. SPIE-Int. Soc. Opt. Eng., 1989, IS 5, 102–111.
R. M. Valentine, Biophysical Aspects of Photodynamic Therapy, PhD Thesis, University of St Andrews, 2011.
M. L. de Jode, Monte Carlo simulations of light distributions in an embedded tumour model: studies of selectivity in photodynamic therapy, Lasers Med. Sci., 2000, 15, 49–56.
L. G. Henyey, and J. L. Greenstein, Diffuse radiation in the galaxy, Astrophys. J., 1941, 93, 70–83.
S. L. Jacques, C. A. Alter, and S. A. Prahl, Angular dependence of HeNe laser light scattering by human dermis, Lasers Life Sci., 1987, 1, 309–333.
I. Pavlova, C. R. Weber, R. A. Schwarz, M. Williams, A. El-Naggar, A. Gillenwater, R. Richards-Kortum, Monte Carlo model to describe depth selective fluorescence spectra of epithelial tissue: applications for diagnosis of oral precancer, J. Biol. Opt., 2008, 13(6), 064012.
K. Wood, J. E. Bjorkman, B. Whitney, and A. D. Code, The effect of multiple scattering on the polarization from axisymmetric circumstellar envelopes. I. Pure Thomson scattering envelopes, Astrophys. J., 1996, 461, 828–846.
K. Wood, J. E. Bjorkman, B. Whitney, and A. D. Code, The effect of multiple scattering on the polarization from axisymmetric circumstellar envelopes. II. Thomson scattering in the presence of absorptive opacity sources, Astrophys. J., 1996, 461, 847–857.
E. Salomatina, B. Jiang, J. Novak, and A. N. Yaroslavsky, Optical properties of normal and cancerous human skin in the visible and near-infrared spectral range, J. Biol. Opt., 2006, 11, 064026.
C. Gardner, S. L. Jacques, and A. J. Welch, Light transport in tissue: accurate expressions for one-dimensional fluence rate and escape function based upon Monte Carlo simulation, Lasers Surg. Med., 1996, 18, 129–138.
A. R. Oseroff, S. Shieh, N. P. Frawley, R. Cheney, L. E. Blumenson, E. K. Pivnick, and D. A. Bellnier, Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy, Arch. Dermatol., 2005, 141, 60–67.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of a themed issue on current topics in photodermatology.
Rights and permissions
About this article
Cite this article
Valentine, R.M., Ibbotson, S.H., Wood, K. et al. Modelling fluorescence in clinical photodynamic therapy. Photochem Photobiol Sci 12, 203–213 (2013). https://doi.org/10.1039/c2pp25271f
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c2pp25271f