Abstract
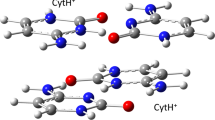
We report on spectral features for two and three diphenylacetylene chromophores aligned in close proximity in aqueous solution by self assembly of attached oligonucleotide arms. Two duplex systems were examined in detail. One was formed by hybridization (Watson—Crick base pairing) of two oligonucleotide 10-mers, each containing the diphenylacetylene insert. The other was generated by self-folding of a 36-mer oligonucleotide containing two diphenylacetylene inserts. The triplex system was obtained by hybridization (Hoogsteen base pairing) of a 16-mer oligonucleotide diphenylacetylene conjugate to the folded 36-mer hairpin. Formation of duplex and triplex entities from these conjugates was demonstrated experimentally by thermal dissociation and spectroscopic studies. The UV and CD spectra for the duplex systems exhibit bands in the 300–350 nm region attributable to exciton coupling between the two chromophores, and the emission spectra show a strong band centered at 410 nm assigned to excimer fluorescence. Addition of the third strand to the hairpin duplex has little effect on the CD spectrum in the 300–350 nm region, but leads to a negative band at short wavelengths characteristic of a triplex and to a strongly enhanced band at 410 nm in the fluorescence spectrum. The third strand alone shows a broad fluorescence band at ~345–365 nm, but this band is virtually absent in the triplex system. A model for the triplex system is proposed in which two of the three aligned diphenylacetylenes function as a ground state dimer that on excitation gives rise to the exciton coupling observed in the UV and CD spectra and to the excimer emission observed in the fluorescence spectrum. Excitation of the third chromophore results in enhanced excimer fluorescence, as a consequence of energy transfer from the locally excited singlet of one chromophore to the ground state dimer formed by the other two chromophores.
Similar content being viewed by others
References
X. Li and D. R. Liu, DNA-Templated organic synthesis: Nature’s strategy for controlling chemical reactivity applied to synthetic molecules, Angew. Chem., Int. Ed., 2004, 43, 4848–4870.
J. Svoboda, B. König, Templated photochemistry: Toward catalysts enhancing the efficiency and selectivity of photoreactions in homogeneous solutions, Chem. Rev., 2006, 106, 5413–5430.
R. L. Letsinger and T. Wu, Control of excimer emission and photochemistry of stilbene units by oligonucleotide hybridization, J. Am. Chem. Soc., 1994, 116, 811–812.
F. D. Lewis, T. Wu, E. L. Burch, D. M. Bassani, J.-S. Yang, S. Schneider, W. Jäger and R. L. Letsinger, Hybrid oligonucleotides containing stilbene units. Excimer fluorescence and photodimerization, J. Am. Chem. Soc., 1995, 117, 8785–92.
R. L. Letsinger, T. F. Wu and R. Elghanian, Chemical and photochemical ligation of oligonucleotide blocks, Nucleosides & Nucleotides, 1997, 16, 643–652.
Y. Zheng, H. Long, G. C. Schatz and F. D. Lewis, Duplex and hairpin dimer structures for perylene diimide–oligonucleotide conjugates, Chem. Commun., 2005, 4795–4797.
R. L. Letsinger and S. Chaturvedi, Tailored hydrophobic cavities in oligonucleotide-steroid conjugates, Bioconjugate Chem., 1998, 9, 826–830.
G. Büchi, C. W. Perry and E. W. Robb, Photochemical reactions. XI. Diphenylacetylene, J. Org. Chem., 1962, 27, 4106–4107.
S. Bevers, S. Schutte and L. W. Mclaughlin, Naphthalene and perylene based linkers for the stabilization of hairpin triplex, J. Am. Chem. Soc., 2000, 122, 5905–5919.
I. Trkulja, R. Häner, Monomeric and heterodimeric triple helical DNA mimics, J. Am. Chem. Soc., 2007, 129, 7982–7989.
F. D. Lewis, X. Liu, S. E. Miller and M. R. Wasielewski, Electronic interactions between p-stacked DNA base pairs and diphenylacetylene-4,4’-dicarboxamide in hairpin DNA, J. Am. Chem. Soc., 1999, 121, 9746–9747.
F. D. Lewis, X. Liu, S. E. Miller, R. T. Hayes and M. R. Wasielewski, Formation and decay of localized contact radical ion pairs in DNA hairpins, J. Am. Chem. Soc., 2002, 124, 14020–14026.
N. Berova, K. Nakanishi and R. W. Woody, Circular Dichroism Wiley-VCH, New York, 2000
A. E. Clark, C. Qin and A. D. Q. Li, Beyond exciton theory: A time-dependent DFT and Franck–Condon study of perylene diimide and its chromophoric dimer, J. Am. Chem. Soc., 2007, 129, 7586–7595.
B. Brocklehurst, D. C. Bull, M. Evans, P. M. Scott and G. Stanney, Excimer fluorescence of trans-stilbene and diphenylacetylene, J. Am. Chem. Soc., 1975, 97, 2977–2978.
F. D. Lewis, Y. Zhang and R. L. Letsinger, Bispyrenyl excimer fluorescence: A sensitive oligonucleotide probe, J. Am. Chem. Soc., 1997, 119, 5451–5452.
C. A. M. Seidel, A. Schulz and M. H. M. Sauer, Nuclobase-specific quenching of fluorescent dyes. 1. Nucleobase one-electron redox potentials and their correlation with static and dynamic quenching efficiencies, J. Phys. Chem., 1996, 100, 5541–5553.
F. D. Lewis, T. Wu, X. Liu, R. L. Letsinger, S. R. Greenfield, S. E. Miller and M. R. Wasielewski, Dynamics of photoinduced charge separation and charge recombination in synthetic DNA hairpins with stilbenedicarboxamide linkers, J. Am. Chem. Soc., 2000, 122, 2889–2902.
S. M. Langenegger, R. Häner, Excimer formation by interstrand stacked pyrenes, Chem. Commun., 2004, 2792–2793.
R. L. Letsinger and T. Wu, Use of a stilbenedicarbxoamide bridge in stabilizing, monitoring, and photochemically altering folded conformations of oligonucleotides, J. Am. Chem. Soc., 1995, 117, 7323–7328.
L. E. Xodo, G. Manzini, F. Quadrifoglio, G. Vandermarel and J. Vanboom, DNA hairpin loops in solution–correlation between primary structure, thermostability and reactivity with single-strand-specific nuclease from mung bean, Nucleic Acids Res., 1991, 19, 1505–1511.
J. SantaLucia, R. Kierzek and D. H. Turner, Context dependence of hydrogen-bond free-energy revealed by substitutions in an RNA hairpin, Science, 1992, 256, 217–219.
E. Wang and J. Feigon, Structures of nucleic acid triplexes, in Nucleic Acid Structure ed. S. Neidle, Oxford, UK, 1999
J. B. Birks, Photophysics of Aromatic Molecules Wiley-Interscience, London, 1970
G. E. Plum and K. J. Breslauer, Thermodynamics of an intramolecular DNA triple-helix - a calorimetric and spectroscopic study of the pH and salt dependence of thermally-induced structural transitions, J. Mol. Biol., 1995, 248, 679–695.
G. E. Plum, Thermodynamics of oligonucleotide triple helices, Biopolymers, 1997, 44, 241–256.
R. W. Roberts and D. M. Crothers, Prediction of the stability of DNA triplexes, Proc. Natl. Acad. Sci. USA, 1996, 93, 4320–4325.
F. D. Lewis, Y. Wu and X. Liu, Synthesis, structure, and photochemistry of exceptionally stable synthetic DNA hairpins with stilbene diether linkers, J. Am. Chem. Soc., 2002, 124, 12165–12173.
D. R. James, A. Siemiarczuk and W. R. Ware, Stroboscopic optical boxcar technique for the determination of fluorescence lifetimes, Rev. Sci. Instrum., 1992, 63, 1710–1716.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Letsinger, R.L., Wu, T., Yang, JS. et al. DNA-Templated formation and luminescence of diphenylacetylene dimeric and trimeric complexes. Photochem Photobiol Sci 7, 854–859 (2008). https://doi.org/10.1039/b805452e
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b805452e