Abstract
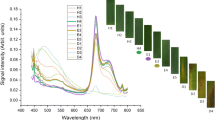
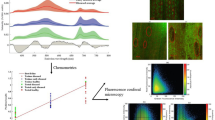
Biotic and abiotic stress both cause a considerable decrease in the chlorophyll content in plant leaves, which provides a means for the early diagnosis of diseases in plants. The emergence of diseases affects the fluorescence of phenolic compounds and chlorophyll, which have emissions located at 530, 686 and 735 nm. Herein, it was found that the intensity of the emission band of phenolic compounds at 530 nm increased and that of chlorophyll at 735 nm decreased with the onset of diseases. Statistical analysis through principal component analysis (PCA) and partial least squares regression (PLSR) was performed, which differentiated between apparently healthy leaf sites and diseased leaves, providing a basis for the detection of diseases in the early stages. The PLSR model was validated through the coefficient of determination (R2), standard error of prediction (SEP) and standard error of calibration (SEC) with the values of 0.99, 0.394 and 0.0.401, respectively, which authenticated the model. The prediction accuracy of the model was evaluated through root mean square error in prediction (RMSEP), with a value of 0.14, by predicting 22 unknown emission spectra of different leaf sites. Both the PCA and PLSR models produced similar results, proving that fluorescence spectroscopy is an excellent tool for early disease detection in plants.
Similar content being viewed by others
References
J. M. Alston, J. M. Beddow and P. G. Pardey, Agricultural Research, Productivity, and Food Prices in the Long Run, Science, 2009, 325, 1209–1210.
H. C. J. Godfray, J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, J. Pretty, S. Robinson, S. M. Thomas and C. Toulmin, Food Security: The Challenge of Feeding 9 Billion People, Science, 2010, 327, 812–818.
R. Pedrós, I. Moya, Y. Goulas and S. Jacquemoud, Chlorophyll fluorescence emission spectrum inside a leaf, Photochem. Photobiol. Sci., 2008, 7, 498–502.
K. Maxwell and G. N. Johnson, Chlorophyll fluorescence - a practical guide, J. Exp. Bot., 2000, 51, 659–668.
B. Anderson, P. K. Buah-Bassuah and J. P. Tetteh, Using violet laser-induced chlorophyll fluorescence emission spectra for crop yield assessment of cowpea (Vigna unguiculata (L) Walp) varieties, Meas. Sci. Technol., 2004, 15, 1255–1265.
M. Pérez-Patricio, J. L. Camas-Anzueto, A. Sanchez-Alegría, A. Aguilar-González, F. Gutiérrez-Miceli, E. Escobar-Gómez, Y. Voisin, C. Rios-Rojas and R. Grajales-Coutiño, Optical method for estimating the chlorophyll contents in plant leaves, Sensors, 2018, 18, 650.
H. Wang, X. Qian, L. Zhang, S. Xu, H. Li, X. Xia, L. Dai, L. Xu, J. Yu and X. Liu, Detecting crop population growth using chlorophyll fluorescence imaging, Appl. Opt., 2017, 56, 9762–9769.
L. Nedbal and J. Whitmarsh, Chlorophyll Fluorescence Imaging of Leaves and Fruits, in Chlorophyll a Fluorescence, Springer, 2004, pp. 389–407.
A. Friedrichs, J. A. Busch, H. J. van der Woerd and O. Zielinski, SmartFluo: A method and affordable adapter to measure chlorophyll a fluorescence with smartphones, Sensors, 2017, 17, 1–14.
S. Lenk, L. Chaerle, E. E. Pfündel, G. Langsdorf, D. Hagenbeek, H. K. Lichtenthaler, D. Van Der Straeten and C. Buschmann, Multispectral fluorescence and reflectance imaging at the leaf level and its possible applications, J. Exp. Bot., 2007, 58, 807–814.
H. K. Lichtenthale and J. A. Miehe, Fluorescence imaging as a diagnostic tool for plant stress, Trends Plant Sci., 1997, 2, 316–320.
H. Wang, X. Qian, L. Zhang, S. Xu, H. Li, X. Xia, L. Dai, L. Xu, J. Yu and X. Liu, A Method of High Throughput Monitoring Crop Physiology Using Chlorophyll Fluorescence and Multispectral Imaging, Front. Plant Sci., 2018, 9, 1–12.
N. Tremblay, Z. Wang and Z. G. Cerovic, Sensing crop nitrogen status with fluorescence indicators. A review, Agron. Sustainable Dev., 2012, 32, 451–464.
J. Yang, L. Du, W. Gong, S. Shi, J. Sun and B. Chen, Potential of vegetation indices combined with laser-induced fluorescence parameters for monitoring leaf nitrogen content in paddy rice, PLoS One, 2018, 13, 1–15.
J. Cubero, J. H. Graham and T. R. Gottwald, Quantitative PCR Method for Diagnosis of Citrus Bacterial Canker, Appl. Environ. Microbiol., 2001, 67, 2849–2852.
J. D. Scholes and S. A. Rolfe, Chlorophyll fluorescence imaging as tool for understanding the impact of fungal diseases on plant performance: a phenomics perspective, Funct. Plant Biol., 2009, 36, 880.
L. Chaerle, D. Hagenbeek, E. De Bruyne, R. Valcke and D. Van Der Straeten, Thermal and Chlorophyll-Fluorescence Imaging Distinguish Plant-Pathogen Interactions at an Early Stage, Plant Cell Physiol., 2004, 45, 887–896.
F. M. V. Pereira, D. M. B. P. Milori, E. R. Pereira-Filho, A. L. Venâncio, M. de S. T. Russo, M. C. do B. Cardinali, P. K. Martins and J. Freitas-Astúa, Laser-induced fluorescence imaging method to monitor citrus greening disease, Comput. Electron. Agric., 2011, 79, 90–93.
J. Belasque Jr., M. C. G. Gasparoto and L. G. Marcassa, Detection of mechanical and disease stresses in citrus plants by fluorescence spectroscopy, Appl. Opt., 2008, 47, 1922.
E. C. Lins, J. Belasque and L. G. Marcassa, Detection of citrus canker in citrus plants using laser induced fluorescence spectroscopy, Precis. Agric., 2009, 10, 319–330.
C. B. Wetterich, R. F. de O. Belasque, N. José and L. G. Marcassa, Detection of citrus canker and Huanglongbing using fluorescence imaging spectroscopy and support vector machine technique, Appl. Opt., 2016, 55, 400–407.
P. F. Daley, Chlorophyll fluorescence analysis and imaging in plant stress and disease, Can. J. Plant Pathol., 1995, 17, 167–173.
R. Hull and R. Hull, Assay, Detection, and Diagnosis of Plant Viruses, in Plant Virology, Elsevier, 2014, pp. 755–808.
B. M. Atta, M. Saleem, H. Ali, H. M. I. Arshad and M. Ahmed, Chlorophyll as a biomarker for early disease diagnosis, Laser Phys., 2018, 28, 065607.
M. D. Brooks and K. K. Niyogi, Use of a Pulse-Amplitude Modulated Chlorophyll Fluorometer to Study the Efficiency of Photosynthesis in Arabidopsis Plants, in Methods in molecular biology, Springer, 2011, vol. 775, pp. 299–310.
U. Schreiber, Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview, in Chlorophyll a Fluorescence, Springer, 2004, pp. 279–319.
T. Dong, J. Shang, J. M. Chen, J. Liu, B. Qian, B. Ma, M. J. Morrison, C. Zhang, Y. Liu, Y. Shi, H. Pan and G. Zhou, Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration, Remote Sens., 2019, 11, 2706.
Y. Goulas, Z. G. Cerovic, A. Cartelat and I. Moya, Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence, Appl. Opt., 2004, 43, 4488–4496.
F. M. Padilla, M. Gallardo, M. T. Peña-Fleitas, R. de Souza and R. B. Thompson, Proximal Optical Sensors for Nitrogen Management of Vegetable Crops: A Review, Sensors, 2018, 18, 2083.
F. M. Padilla, R. de Souza, M. T. Peña-Fleitas, M. Gallardo, C. Giménez and R. B. Thompson, Different Responses of Various Chlorophyll Meters to Increasing Nitrogen Supply in Sweet Pepper, Front. Plant Sci., 2018, 9, 1752.
Y. Li and M. Chen, Novel chlorophylls and new directions in photosynthesis research, Funct. Plant Biol., 2015, 42, 493–501.
J. P. Palta, Leaf chlorophyll content, Remote Sens. Rev., 1990, 5, 207–213.
A. V. Ruban, Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage, Plant Physiol., 2016, 170, 1903–1916.
M. Lang, F. Stober and H. K. Lichtenthaler, Fluorescence emission spectra of plant leaves and plant constituents, Radiat. Environ. Biophys., 1991, 30, 333–347.
J. M. Harnly, S. Bhagwat and L. Z. Lin, Profiling methods for the determination of phenolic compounds in foods and dietary supplements, Anal. Bioanal. Chem., 2007, 389, 47–61.
A. A. Gitelson, C. Buschmann and H. K. Lichtenthaler, Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements, J. Plant Physiol., 1998, 152, 283–296.
A. A. Gitelson, C. Buschmann and H. K. Lichtenthaler, The Chlorophyll Fluorescence Ratio F735/F700 as an Accurate Measure of the Chlorophyll Content in Plants, Remote Sens. Environ., 1999, 69, 296–302.
K. Yu, G. Leufen, M. Hunsche, G. Noga, X. Chen and G. Bareth, Investigation of Leaf Diseases and Estimation of Chlorophyll Concentration in Seven Barley Varieties Using Fluorescence and Hyperspectral Indices, Remote Sens., 2013, 6, 64–86.
G. Agati, P. Mazzinghi, F. Fusi and I. Ambrosini, The F685/F730 Chlorophyll Fluorescence Ratio as a Tool in Plant Physiology: Response to Physiological and Environmental Factors, J. Plant Physiol., 1995, 145, 228–238.
E. V. Thomas and D. M. Haaland, Comparison of multivariate calibration methods for quantitative spectral analysis, Anal. Chem., 1990, 62, 1091–1099.
S. N. Jha and R. Garg, Non-destructive prediction of quality of intact apple using near infrared spectroscopy, J. Food Sci. Technol., 2010, 47, 207–213.
S. L. Cantor, S. W. Hoag, C. D. Ellison, M. A. Khan and R. C. Lyon, NIR spectroscopy applications in the development of a compacted multiparticulate system for modified release, AAPS PharmSciTech, 2011, 12, 262–278.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c9pp00368a
Rights and permissions
About this article
Cite this article
Saleem, M., Atta, B.M., Ali, Z. et al. Laser-induced fluorescence spectroscopy for early disease detection in grapefruit plants. Photochem Photobiol Sci 19, 713–721 (2020). https://doi.org/10.1039/c9pp00368a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c9pp00368a