Abstract
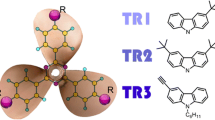
The photophysical properties of a novel series of non-homoconjugated 1,2-di-, 1,2,3,4-tetra-, and 1,2,3,4,5,6-hexasubstituted fullerenes (compounds 1, 2, and 3, respectively) have been systematically investigated. In this report, we examine the effect of substitution pattern of non-homoconjugated derivatized fullerenes on the ground state UV-Vis absorption, triplet state properties (lifetime, quantum yield, extinction coefficient), and singlet oxygen quantum yield. The non-homoconjugated fullerene derivatives 1-3 exhibit higher singlet oxygen quantum yield than analogous homoconjugated Bingel adducts with the same number of saturated C=C bonds and exhibit decreasing quantum yield of singlet oxygen generation upon increasing the degree of functionalization on a single six member ring on the fullerene cage. This trend is similar for triplet quantum yield and triplet lifetime. The triplet extinction coefficient increases with functionalization. A detailed discussion comparing 1, 2, and 3 with functionalized homoconjugated systems and with other non-homoconjugated derivatives is presented.
Similar content being viewed by others
References
S. Anderson, H. L. Anderson, J. K. M. Sanders, Expanding roles for templates in synthesis, Acc. Chem. Res., 1993, 26, 469–475.
L. Isaacs, R. F. Haldimann, F. Diederich, Tether-directed remote functionalization of buckminsterfullerene: regiospecific hexaadduct formation, Angew. Chem., Int. Ed. Engl., 1994, 33, 2339–2342.
S. Sergeyev, F. Diederich, Regio- and stereoselective tether-directed remote functionalization of C60 with derivatives of the Tröger base, Angew. Chem., Int. Ed. Engl., 2004, 43, 1738–1740.
I. Lamparth, C. Maichle-Mössner, A. Hirsch, Reversible template-directed activation of equatorial double bonds of the fullerene framework: regioselective direct synthesis, crystal structure, and aromatic properties of Th-C66(COOEt)12, Angew. Chem., Int. Ed. Engl., 1995, 34, 1607–1609.
F. Diederich, M. Gomez-Lopez, Supramolecular fullerene chemistry, Chem. Soc. Rev., 1999, 28, 263–277.
U. Reuther, T. Brandmüller, W. Donaubauer, F. Hampel, A. Hirsch, A highly regioselective approach to multiple adducts of C60 governed by strain minimization of macrocyclic malonate addends, Chem.-Eur. J., 2002, 8, 2261–2273.
W. Qian, Y. Rubin, Convergent, regioselective synthesis of tetrakisfulleroids from C60, J. Org. Chem., 2002, 67, 7683–7687.
C. Thilgen, F. Diederich, Tether-directed remote functionalization of fullerenes C60 and C70, C. R. Chim., 2006, 9, 868–880.
A. Hirsch, I. Lamparth, H. R. Karfunkel, Fullerene chemistry in three dimensions: isolation of seven regioisomeric bisadducts and chiral trisadducts of C60and di(ethoxycarbonyl)methylene, Angew. Chem., Int. Ed. Engl., 1994, 33, 437–438.
C. Bingel, Cyclopropanierung von fullerenen, Chem. Ber., 1993, 126, 1957–1959.
S. R. Wilson, Q. Lu, 2,6-Dimethoxyanthracene - a directing group for regioselective bisaddition to C60, Tetrahedron Lett., 1995, 36, 5707–5710.
A. Duarte-Ruiz, T. Müller, K. Wurst, B. Kräutler, The bis-adducts of the [5,6]-fullerene C60 and anthracene, Tetrahedron, 2001, 57, 3709–3714.
M. Maggini, G. Scorrano, M. Prato, Addition of azomethine ylides to C60: synthesis, characterization, and functionalization of fullerene pyrrolidines, J. Am. Chem. Soc., 1993, 115, 9798–9799.
S. R. Wilson, Y. Wang, J. Cao, X. Tan, Amino acids as precursors for N-unsubstituted fulleropyrrolidine derivatives, Tetrahedron Lett., 1996, 37, 775–778.
Q. Lu, D. I. Schuster, S. R. Wilson, Preparation and characterization of six bis(N-methylpyrrolidine)-C60 isomers: magnetic deshielding in isomeric bisadducts of C60, J. Org. Chem., 1996, 61, 4764–4768.
Y. Nakamura, N. Takano, T. Nishimura, E. Yashima, M. Sato, T. Kudo, N. Nishimura, First isolation and characterization of eight regioisomers for [60]fullerene-benzyne bisadducts, Org. Lett., 2001, 3, 1193–1196.
F. Diederich, R. Kessinger, Templated regioselective and stereoselective synthesis in fullerene chemistry, Acc. Chem. Res., 1999, 32, 537–545.
F. Djojo, A. Herzog, I. Lamparth, F. Hampel, A. Hirsch, Regiochemistry of two-fold additions to [6,6] bonds in C60: influence of the addend-independent cage distortion in 1,2-monoadducts, Chem.-Eur. J., 1996, 2, 1537–1547.
G. Shick, A. Hirsch, H. Mauser, T. Clark, Opening and closure of the fullerene cage in cis-bisimino adducts of C60: The influence of the addition pattern and the addend, Chem.-Eur. J., 1996, 2, 935–943.
M. W. J. Beulen, J. A. Rivera, M. A. Herranz, B. Illescas, N. Martín, L. Echegoyen, Reductive electrochemistry of spiromethanofullerenes, J. Org. Chem., 2001, 66, 4393–4398.
T. Hamano, K. Okuda, T. Mashino, M. Hirobe, K. Arakane, A. Ryu, S. Mashiko, T. Nagano, Singlet oxygen production from fullerene derivatives: effect of sequential functionalization of the fullerene core, Chem. Commun., 1997, 1, 21–22.
F. Prat, R. Stackow, R. Bernstein, W. Y. Qian, Y. Rubin, C. S. Foote, Triplet-state properties and singlet oxygen generation in a homologous series of functionalized fullerene derivatives, J. Phys. Chem. A, 1999, 103, 7230–7235.
Y. Nakamura, M. Taki, S. Tobita, H. Shizuka, H. Yokoi, K. Ishiguro, Y. Sawaki, J. Nishimura, Photophysical properties of various regioisomers of [60]fullerene- o-quinodimethane bisadducts, J. Chem. Soc., Perkin Trans. 2, 1999, 1, 127–130.
P. F. Coheur, D. A. dos Santos, J. Cornil, P. R. Birkett, J. Liévin, J. L. Brédas, D. R. M. Walton, R. Taylor, H. W. Kroto, R. Colin, Photophysical properties of hexa-functionalized C60 derivatives: Spectroscopic and quantum-chemical investigations, J. Chem. Phys., 2000, 112, 8555–8566.
S. Foley, S. Bosi, C. Larroque, M. Prato, J. M. Janot, P. Seta, Photophysical properties of novel water soluble fullerene derivatives, Chem. Phys. Lett., 2001, 350, 198–205.
J. W. Arbogast, C. S. Foote, Photophysical properties of C70, J. Am. Chem. Soc., 1991, 113, 8886–8889.
U. Reuther, T. Brandmüller, W. Donaubauer, F. Hampel, A. Hirsch, A highly regioselective approach to multiple adducts of C60 governed by strain minimization of macrocyclic malonate addends, Chem.-Eur. J., 2002, 8, 2261–2273.
K. K. Chin, S.-C. Chuang, B. Hernandez, M. Selke, C. S. Foote, M. A. Garcia-Garibay, Photophysical properties of a 1,2,3,4,5,6-hexasubstituted fullerene derivative, J. Phys. Chem. A, 2006, 110, 13662–13666.
S.-C. Chuang, M. Sander, T. Jarrosson, S. James, E. Rozumov, S. I. Khan, Y. Rubin, Approaches to open fullerenes: synthesis and kinetic stability of Diels-Alder adducts of substituted isobenzofurans and C60, J. Org. Chem., 2007, 72, 2716–2723.
M. Sander, T. Jarrosson, S.-C. Chuang, S. I. Khan, Y. Rubin, Approaches to open fullerenes: synthesis and thermal stability of cis-1 bis(isobenzofuran) Diels-Alder adducts of C60, J. Org. Chem., 2007, 72, 2724–2731.
S.-C. Chuang, F. R. Clemente, S. I. Khan, K. N. Houk, Y. Rubin, Approaches to open fullerenes: a 1,2,3,4,5,6-hexaadduct of C60, Org. Lett., 2006, 8, 4525–4528.
D. M. Guldi, K.-D. Asmus, Photophysical properties of mono- and multiply-functionalized fullerene derivatives, J. Phys. Chem. A, 1997, 101, 1472–1481.
K. George Thoma, V. Biju, M. V. George, Excited-state interactions in pyrrolidinofullerenes, J. Phys. Chem. A, 1998, 102, 5341–5348.
The MER Corp., 7960 S. Kolb Road, Tucson, AZ 85706
R. N. Warrener, Isolation of isobenzofuran, a stable but highly reactive molecule, J. Am. Chem. Soc., 1971, 93, 2346–2348.
W. Friedrichsen, Adv. Heterocycl. Chem., 1980, 26, 135–241.
R. Rodrigo, Progress in the chemistry of isobenzofurans: Applications to the synthesis of natural products and polyaromatic hydrocarbons, Tetrahedron, 1988, 44, 2093–2135.
B. Rickborn, in Advances in Theoretically Interesting Molecules, ed. R. P. Thummel, JAI Press Inc., Greenwich, CT, 1989, vol. I, pp. 1–134.
C. W. Bird, Prog. Heterocycl. Chem., 1990, 2, 87–101.
O. Peters, W. Friedrichsen, Trends Heterocycl. Chem., 1995, 4, 217–259.
I. Carmichael, G. L. Hug, Triplet-triplet absorption spectra of organic molecules in condensed phases, J. Phys. Chem. Ref. Data, 1986, 15, 1–250.
S. L. Murov, I. Carmichael and G. L. Hug, Handbook of Photochemistry, Marcel Dekker, New York, 1993.
J. L. Anderson, Y.-Z. An, Y. Rubin, C. S. Foote, Photophysical characterization and singlet oxygen yield of a dihydrofullerene, J. Am. Chem. Soc., 1994, 116, 9763–9764.
F. Prat, R. Stackow, R. Bernstein, W. Y. Qian, Y. Rubin, C. S. Foote, Triplet-state properties and singlet oxygen generation in a homologous series of functionalized fullerene derivatives, J. Phys. Chem. A, 1999, 103, 7230–7235.
R. V. Bensasson, E. Bienvenue, J. M. Janot, S. Leach, P. Seta, D. I. Schuster, S. R. Wilson, H. Zhao, Photophysical properties of three hydrofullerenes, Chem. Phys. Lett., 1995, 245, 566–570.
C. Lee, W. Yang, R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789.
A. D. Becke, Density-functional thermochemistry. III. The, role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, GAUSSIAN 03 (Revision C.02), Gaussian, Inc., Wallingford, CT, 2004.
W. Qian, M. D. Bartberger, S. J. Pastor, K. N. Houk, C. L. Wilkins, Y. Rubin, C62, a non-classical fullerene incorporating a four-membered ring, J. Am. Chem. Soc., 2000, 122, 8333–8334, and references therein
C. Schweitzer, R. Schmidt, Physical, mechanisms of generation and deactivation of singlet oxygen, Chem. Rev., 2003, 103, 1685–1757.
O. L. Gijzeman, F. Kaufman, G. Porter, Oxygen, quenching of aromatic triplet states in solution, J. Chem. Soc. Faraday Trans. 2, 1973, 69, 708–720.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chin, K.K., Chuang, SC., Hernandez, B. et al. Photophysical properties of non-homoconjugated 1,2-dihydro, 1,2,3,4-tetrahydro and 1,2,3,4,5,6-hexahydro-C60 derivatives. Photochem Photobiol Sci 7, 49–55 (2008). https://doi.org/10.1039/b714076b
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/b714076b