The relevance of establishing the structure of electrolyte solutions of low dielectric permittivity is briefly recalled. Microwave complex dielectric permittivities ε* = ε′ − Jε″ in the frequency range ∼1 to 130 GHz for LiClO4 dissolved in poly(ethylene oxide)dimethyl ether of average molar mass 500 (PEO-500), in the concentration range 0.40 to 1 mol dm−3, at 25 °C, are reported. The data are interpreted by a Cole–Davidson distribution function. Molar electrical conductance for the same system, in the concentration range 7 × 10−4 to ∼1 mol dm−3, shows a minimum and a maximum as a function of the electrolyte concentration. The data, in the diluted range (⩽0.017 mol/dm3) are interpreted by the Fuoss–Onsager equation, in terms of free ions and ion-pair-species. Ultrasonic relaxation spectra in the frequency range 1 to 300 MHz and concentration range 0.1 to ∼0.4 mol dm−3, are interpreted by the ion-pair dimerization equilibrium 2LiClO4 ↔ (LiClO4)2, the dimer being an apolar antiparallel species 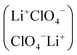 , thus rationalizing the depolarization of the solution with the consequent appearance of a maximum in the dielectric permittivity of the solution at high electrolyte concentration. A parallel with previous work on LiPF6 dissolved in the same solvent is drawn.
, thus rationalizing the depolarization of the solution with the consequent appearance of a maximum in the dielectric permittivity of the solution at high electrolyte concentration. A parallel with previous work on LiPF6 dissolved in the same solvent is drawn.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
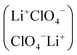 , thus rationalizing the depolarization of the solution with the consequent appearance of a maximum in the dielectric permittivity of the solution at high electrolyte concentration. A parallel with previous work on LiPF6 dissolved in the same
, thus rationalizing the depolarization of the solution with the consequent appearance of a maximum in the dielectric permittivity of the solution at high electrolyte concentration. A parallel with previous work on LiPF6 dissolved in the same 
 Please wait while we load your content...
Please wait while we load your content...