Abstract
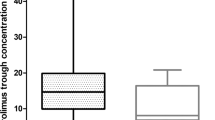
The objective of this study was to determine the effect of the CYP3A5 and ATP binding cassette subfamily B member 1 (ABCB1) single-nucleotide polymorphisms on the disposition of sunitinib and SU12662, on clinical response, and on the manifestation of toxicities in Asian metastatic renal cell carcinoma patients. At week 4 of each treatment cycle, toxicities and plasma steady-state levels were assessed. Clinical response was assessed after two cycles. Genotyping was performed by using the PCR restriction fragment length polymorphism method. The CC genotype for ABCB1 was associated with a higher sunitinib exposure (76.81 vs 56.55 ng ml−1, P=0.03), higher risk of all-grade rash (RR 3.00, 95% CI 1.17–7.67) and mucositis (RR 1.60, 95% CI 1.10–2.34) and disease progression than compared with the CT/TT genotype. There was a lack of association observed between the CYP3A5 polymorphism and exposure, response and toxicities. The polymorphism of ABCB1 (C3435T) has an important role in the manifestation of toxicities and drug exposure, but not polymorphism of CYP3A5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pfizer. Sutent Prescribing Information 2012.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O et al. Sunitinib versus Interferon Alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–124.
Clinical Practice Guidelines in Oncology, Kidney Cancer. National Comprehensive Cancer Network (NCCN) 2014. Available from www.NCCN.org.
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 2009; 10: 757–763.
Yoo C, Kim JE, Lee JL, Ahn JH, Lee DH, Lee JS et al. The efficacy and safety of sunitinib in Korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol 2010; 40: 980–985.
Lee SH, Bang YJ, Mainwaring P, Ng C, Chang JWC, Kwong P et al. Sunitinib in metastatic renal cell carcinoma: an ethnic asian subpopulation analysis for safety and efficacy. Asia Pac J Clin Oncol 2014; 10: 237–245.
Yu H, Steeghs N, Nijenhuis CM, Schellens JHM, Beijnen JH, Huitema ADR . Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet 2014; 53: 305–325.
Houk BE, Bello CL, Kang D, Amantea M . A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 2009; 15: 2497–2506.
Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ . Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: Results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 2010; 66: 357–371.
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 2001; 27: 383–391.
Sugiyama M, Fujita K, Murayama N, Akiyama Y, Yamazaki H, Sasaki Y . Sorafenib and sunitinib, two anticancer drugs, inhibit CYP3A4-mediated and activate CY3A5-mediated midazolam 1'-hydroxylation. Drug Metab Dispos 2011; 39: 757–762.
Balram C, Zhou Q, Cheung YB, Lee EJD . CYP3A5*3 and *6 single nucleotide polymorphisms in three distinct Asian populations. Eur J Clin Pharmacol 2003; 59: 123–126.
Teo YL, Chong XJ, Chue XP, Chau NM, Tan MH, Kanesvaran R et al. Role of sunitinib and SU12662 on dermatological toxicities in metastatic renal cell carcinoma patients: In vitro in vivo, and outcomes investigation. Cancer Chemother Pharmacol 2014; 73: 381–388.
Hoffmeyer S, Burk O, Von Richter O, Arnold HP, Brockmöller J, Johne A et al. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000; 97: 3473–3478.
Beuselinck B, Lambrechts D, Van Brussel T, Wolter P, Cardinaels N, Joniau S et al. Efflux pump ABCB1 single nucleotide polymorphisms and dose reductions in patients with metastatic renal cell carcinoma treated with sunitinib. Acta Oncol 2014; 53: 1413–1422.
Diekstra MHM, Klümpen HJ, Lolkema MPJK, Yu H, Kloth JSL, Gelderblom H et al. Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU12662. Clin Pharmacol Ther 2014; 96: 81–89.
Common Terminology Criteria for Adverse Events (CTCAE). National Institutes of Health, National Cancer Institute 2009.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247.
Etienne-Grimaldi MC, Renee N, Izzedine H, Milano G . A routine feasible HPLC analysis for the anti-angiogenic tyrosine kinase inhibitor, sunitinib, and its main metabolite, SU12662, in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 3757–3761.
Fukuen S, Fukuda T, Maune H, Ikenaga Y, Yamamoto I, Inaba T et al. Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5* 3 and * 6, in a Japanese population. Pharmacogenetics 2002; 12: 331–334.
Cizmarikova M, Wagnerova M, Schonova L, Habalova V, Kohut A, Linkova A et al. MDR1 (C3435T) polymorphism: Relation to the risk of breast cancer and therapeutic outcome. Pharmacogenomics J 2010; 10: 62–69.
Balram C, Sharma A, Sivathasan C, Lee EJ . Frequency of C3435T single nucleotide MDR1 genetic polymorphism in an Asian population: phenotypic-genotypic correlates. Br J Clin Pharmacol 2003; 56: 78–83.
Garcia-Donas J, Esteban E, Leandro-García LJ, Castellano DE, del Alba AG, Climent MA et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol 2011; 12: 1143–1150.
De Wit D, Gelderblom H, Sparreboom A, Den Hartigh J, Den Hollander M, König-Quartel JMC et al. Midazolam as a phenotyping probe to predict sunitinib exposure in patients with cancer. Cancer Chemother Pharmacol 2014; 73: 87–96.
Elens L, Van Gelder T, Hesselink DA, Haufroid V, Van Schaik RHN . CYP3A4*22: promising newly identified CYP3A4 variant allele for personalizing pharmacotherapy. Pharmacogenomics 2013; 14: 47–62.
Leschziner GD, Andrew T, Pirmohamed M, Johnson MR . ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenomics J 2007; 7: 154–179.
Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther 2001; 70: 189–199.
Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T et al. Effect of the mutation (C3435t) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther 2002; 71: 297–303.
Illmer T, Schuler US, Thiede C, Schwarz UI, Kim RB, Gotthard S et al. MDR1 gene polymorphisms affect therapy outcome in acute myeloid leukemia patients. Cancer Res 2002; 62: 4955–4962.
Siegsmund M, Brinkmann U, Schäffeler E, Weirich G, Schwab M, Eichelbaum M et al. Association of the P-glycoprotein transporter MDR1C3435T polymorphism with the susceptibility to renal epithelial tumors. J Am Soc Nephrol 2002; 13: 1847–1854.
Hitzl M, Drescher S, Van der Kuip H, Schäffeler E, Fischer J, Schwab M et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics 2001; 11: 293–298.
Wang D, Johnson AD, Papp AC, Kroetz DL, Sadée W . Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics 2005; 15: 693–704.
Kim HR, Park HS, Kwon WS, Lee JH, Tanigawara Y, Lim SM et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol 2013; 72: 825–835.
Mizuno T, Fukudo M, Fukuda T, Terada T, Dong M, Kamba T et al. The effect of ABCG2 genotype on the population pharmacokinetics of sunitinib in patients with renal cell carcinoma. Ther Drug Monit 2014; 36: 310–316.
Acknowledgements
We acknowledge the Singapore Cancer Society for the provision of grant support for this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Drs Kanesvaran, Wee and Chan serve as consultants to GlaxoSmithKline (Kanesvaran and Chan), Pfizer (Hwee Lin Wee), Novartis Asia Pacific (Hwee Lin Wee) and Abbvie Pte (Hwee Lin Wee). The remaining authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Teo, Y., Wee, H., Chue, X. et al. Effect of the CYP3A5 and ABCB1 genotype on exposure, clinical response and manifestation of toxicities from sunitinib in Asian patients. Pharmacogenomics J 16, 47–53 (2016). https://doi.org/10.1038/tpj.2015.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.13
This article is cited by
-
Is there association between clinically relevant toxicities of pazopanib and sunitinib with the use of weak CYP3A4 and P-gp inhibitors?
European Journal of Clinical Pharmacology (2021)
-
Interaction between phytotherapy and oral anticancer agents: prospective study and literature review
Medical Oncology (2019)
-
Sunitinib-induced hypertension in CYP3A4 rs4646437 A-allele carriers with metastatic renal cell carcinoma
The Pharmacogenomics Journal (2017)
-
Association of Single Nucleotide Polymorphisms in STAT3 with Hand-Foot Skin Reactions in Patients with Metastatic Renal Cell Carcinoma Treated with Multiple Tyrosine Kinase Inhibitors: A Retrospective Analysis in Japanese Patients
Targeted Oncology (2016)