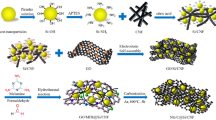
Abstract
Cobalt oxide that has high energy density, is the next-generation candidate as the anode material for LIBs. However, the practical use of Co3O4 as anode material has been hindered by limitations, especially, low electrical conductivity and pulverization from large volume change upon cycling. These features lead to hindrance to its electrochemical properties for lithium-ion batteries. To improve electrochemical properties, we synthesized one-dimensional (1-D) Co3O4 nanofibers (NFs) overed with reduced graphene oxide (rGO) sheets by electrostatic self-assembly (Co3O4 NFs@rGO). The flexible graphene oxide sheets not only prevent volume changes of active materials upon cycling as a clamping layer but also provide efficient electrical pathways by three-dimensional (3-D) network architecture. When applied as an anode for LIBs, the Co3O4 NFs@rGO exhibits superior electrochemical performance: (i) high reversible capacity (615 mAh g−1 and 92% capacity retention after 400 cycles at 4.0 A g−1) and (ii) excellent rate capability. Herein, we highlighted that the enhanced conversion reaction of the Co3O4 NFs@rGO is attributed to effective combination of 1-D nanostructure and low content of rGO (~3.5 wt%) in hybrid composite.
Similar content being viewed by others
Introduction
Lithium-ion batteries (LIBs) have been widely used as the energy source of various devices including portable electronics, electric vehicles (EVs) and energy storage system (ESS) due to their high power, energy density and clean system1,2,3. However, the performance of LIBs is still falling short of the level required for large-scale applications. It pushes the engineers to develop high capacity electrode materials, especially anode, for replacing commercial anode material (e.g. graphite with low specific capacity of 372 mAh g−1). Transition metal oxides (TMOs) introduced by Tarascon’s group are next-generation anode materials thanks to their high theoretical capacity based on conversion reaction4,5,6,7. Among them, cobalt oxide (Co3O4) is an emerging candidate because of its high theoretical capacity (890 mAh g−1), based on the conversion reaction. Typically, during discharge process, Co3O4 can react with 8 moles of lithium ions (Li+) and 8 moles of electrons and form cobalt (Co) metal nanograins which is distributed in lithium oxide (Li2O) matrix. The conversion reaction of Co3O4 is represented in the following equation.

With many Li+ transport and electron transfer, higher theoretical capacity can be obtained from Co3O4 compared to other carbonaceous materials.
Nevertheless, practical use of Co3O4 has been frustrated by the following main challenges: large volume changes of Co3O4 (~300%) upon cycling, low electrical conductivity of Co3O4, and formation of insulative Li2O matrix due to the conversion reaction. In order to address these issues, researchers have often employed nano-engineering to develop Co3O4/carbon nanocomposites such as Co3O4 nanoparticles (NPs)/graphene8, Co3O4 NPs/carbon tube9 and Co3O4 nanowires (NWs)/graphene frame10. Much research has shown that the graphene greatly provides facile passage for electrons and flexible clamping component for Co3O411,12,13. However, Co3O4/graphene composites developed so far require large amount of graphene for stable cycling in LIBs, which sacrifices the actual capacity of Co3O4. Thus, we suggested desirable design for hybrid nanocomposite architecture of Co3O4 and graphene.
One-dimensional (1-D) NFs, in common with other nanostructures, have large electrode/electrolyte interface area with volume-strain relaxation which can prevent the volume expansion during charge/discharge process. Moreover, these NFs have larger electrochemical active area because they are less likely to undergo aggregation than nanoparticles14,15,16,17. In general, large amount of graphene is required to cover nanoparticles with large specific surface area. Meanwhile, it is possible to coat entire surfaces of NFs with very low-content of graphene.
In this work, the hybrid Co3O4 NFs@rGO offer significant benefits that are enumerated briefly as follows: 1-D reinforcement scaffold of the Co3O4 NFs for fast Li+ diffusion, large area for electrode/electrolyte interface, continuous electric contact and volume accommodation, and flexible yet robust graphene sheets directly coated on the Co3O4 NFs for highly electrically conductive networks before and after conversion reactions. Small amount of the rGO greatly improved the performance of nanocomposite, synergistic effect of which on conversion reaction is emphasized.
Results
Synthesis of 1-D Co3O4 NFs and Co3O4 NFs@rGO
As shown in Fig. 1, the 1-D Co3O4 NFs were fabricated via electrospinning method. As-spun Co precursor/polymer composite NFs (as-spun Co(Ac)2/PVP NFs) were achieved after the electrospinning. In subsequent calcination step at 600 °C in air, residual solvent and polymer were burned out and Co precursor was oxidized to Co3O4, resulting in the formation of 1-D Co3O4 NFs. Finally, to cover the surface of the Co3O4 NFs with highly conducting graphene sheets, we employed chemical solution method based on electrostatic interaction and chemical bonds18. The as-prepared Co3O4 NFs were placed in poly(allylamine hydrochloride) solution (PAH solution), where hydroxyl group (-OH) on Co3O4 NFs was modified to amine group (-NH2). These amine groups on the PAH-modified Co3O4 NFs have the electrostatic attraction with the epoxy groups of graphene oxide (GO), and ring opening reaction between the PAH-modified Co3O4 NFs and GO occurs. After reduction of the GO with hydrazine, strong chemical bonds between the Co3O4 NFs and reduced GO (rGO) were generated, leading to the rGO sheets wrapped Co3O4 NFs (Co3O4 NFs@rGO).
Characteristics of Co3O4 NFs and Co3O4@rGO NFs
Morphological features of the Co3O4 NFs@rGO are represented in Fig. 2. The as-spun Co(Ac)2/PVP NFs with smooth and uniform surface were observed in Fig. 2a. During subsequent calcination changing the as-spun NFs into the Co3O4 NFs, the oxidation with shrinkage by burning of residual solvent and polymer was taken place. The calcined Co3O4 NFs were formed in a diameter of 150 to 200 nm (Fig. 2b). After graphene wrapping process, it is clearly observed that the rGO layers are homogeneously coated on the surface of the Co3O4 NFs (Fig. 2c). The internal morphology and phase of the Co3O4 NFs and the Co3O4 NFs@rGO were confirmed by transmission electron microscopy (TEM) (Fig. 2d–f and Fig. S1, see Supplementary Information). The high-resolution TEM (HRTEM) images of the Co3O4 NFs@rGO is shown in Fig. 2e,f, showing that polycrystalline Co3O4 grains connected together were uniformly covered by the ultrathin rGO sheets (~3 nm) (Fig. 2d,e). The lattice fringe of the Co3O4 in the Co3O4 NFs@rGO is 4.66 Å, which is well matched with the spacing of Co3O4 (111) planes (JCPDS PDF#43-1467) (Fig. 2f). The scanning TEM-energy dispersive spectroscopic (STEM-EDS) mapping analysis for the Co3O4 NFs@rGO confirms homogeneous atomic distribution of Co and C in the 1-D scaffold (Fig. 2g,h).
SEM images of (a) the as-spun NFs, (b) the Co3O4 NFs, and (c) the Co3O4 NFs@rGO. (d–f) HRTEM images of the Co3O4 NFs@rGO. (g) STEM image of the Co3O4 NFs@rGO. (h) EDS-mapping image for atomic distribution of Co and C analyzed from the STEM image in (g). (i) XRD patterns of the Co3O4 NFs@rGO, the Co3O4 NFs and JCPDS #42-1467.
To check exact content of carbon in the Co3O4 NFs@rGO, we conducted element analysis (EA). From the EA results, the concentration of carbon was measured to be only 3.56 wt% (Table S1). This value indicates very small amount of carbon in the Co3O4 NFs@rGO. Even using minimum amount of carbon to anode, rGO directly coated on Co3O4 NFs enhances the electrical conductivity and provides high capacity for the Co3O4 NFs. X-ray diffraction (XRD) patterns show that the Co3O4 NFs and the Co3O4 NFs@rGO have the single phase spinel crystal structure for Co3O4 phase (Fig. 2i). The XRD patterns exhibit additional peaks at 2θ = 31.3°, 36.8°, 38.5°, 44.8°, 55.7°, 59.4° and 65.2°, which are attributed to scattering from the (220), (311), (222), (400), (422), (511) and (440) lattice planes of cubic spinel Fd-3m Co3O4 phase (JCPDS PDF#43-1467), respectively. Moreover, any peak change was not observed in the Co3O4 NFs@rGO, indicating that the crystal structure of the Co3O4 NFs is not affected during chemical-solution based graphene wrapping process.
In order to investigate the characteristics of two-dimensional (2-D) graphene layer, Raman spectroscopy analysis was performed. In Raman spectra, five characteristic bands at 195, 478, 517, 618, and 687 cm−1 are discovered in common with three samples (the Co3O4 NFs, PAH-treated Co3O4 NFs and the Co3O4 NFs@rGO) (Fig. 3). The band at 195 cm−1 (F2g) exhibits CoO6 scissoring vibration and other bands at 478, 517, 618 and 687 cm−1 are assigned to Eg, 2F2g and A1g, that exhibit Co-O symmetric stretching vibration, respectively19. In the Co3O4 NFs@rGO, two specific bands were detected at 1356 and 1590 cm−1, which correspond to the D and G band, respectively, confirming that the rGO sheets were formed in the Co3O4 NFs@rGO20. Also, the Raman analysis clearly shows that any structural changes of the Co3O4 NFs did not occur during graphene wrapping step through any changes in peaks of Co3O4.
Electrochemical reaction with Li+ of the Co3O4 NFs and Co3O4 NFs@rGO
The electrochemical performances of the Co3O4 NFs and the Co3O4 NFs@rGO were investigated to check their potential as high-performance anode materials for LIBs. For the electrochemical data, all capacities in this work were based on the weight of all active materials including rGO. The cyclic voltammetric (CV) curves and galvanostatic charge-discharge curves of the Co3O4 NFs and the Co3O4 NFs@rGO are shown in Fig. 4. In Fig. 4a,b the CV graphs show large cathodic peaks at 0.86 V for the Co3O4 NFs and 0.72 V for the Co3O4 NFs@rGO in first cycle. These peaks are attributed to the formation of solid-electrolyte interphase (SEI) layer on the surface of active materials. After the 1st cycle, large cathodic peak at 1.2 V is ascribed to the reduction of Co3O4 by the conversion reaction. The anodic peaks are observed at 2.0 V for both of electrodes, which is ascribed to the oxidation from Co metal nanograins to Co3O421,22. These reaction peaks were well-matched with potentials represented with the plateaus in charge-discharge curves (Fig. 4c,d). The charge-discharge behaviors in the formation cycle, 1st, 10th and 20th cycle for the Co3O4 NFs and the Co3O4 NFs@rGO were observed with voltage window between 0.01 and 3.0 V at current densities of 0.1 A g−1 for formation cycle and 1.0 A g−1 after formation cycle. The discharge capacity in formation cycle of the Co3O4 NFs@rGO shows higher capacity of 1474 mAh g−1, than the Co3O4 NFs (1202 mAh g−1). The irreversible capacity losses of both the Co3O4 NFs and the Co3O4 NFs@rGO were estimated to be 25% of the initial discharge capacity.
Discussion
In general, Co3O4 converts to the Co metal nanograins dispersed in Li2O matrix after conversion reaction with 8 moles of Li+ per one mole of Co3O4. To investigate stability of the the Co3O4 NFs and the Co3O4 NFs@rGO toward such severe reaction with Li+, cycling performances and rate-capability were evaluated (Fig. 5a–c). In Fig. 5a, the Co3O4 NFs show the discharge capacity of 639.4 mAh g−1 in first cycle at a current density of 1.0 A g−1, whereas the discharge capacity of the Co3O4 NFs@rGO is 933.6 mAh g−1 at the same current. For understanding of how the 1-D nanostructures influence their cyclability, we compared the zero-dimensional (0-D) Co3O4 NPs (control sample) with the 1-D Co3O4 NFs and the 1-D Co3O4 NFs@rGO (Fig. S3,SI). Both of the Co3O4 NFs and the Co3O4 NFs@rGO show high capacities that could be maintained up to 200th cycle, whereas the Co3O4 NPs were rapidly degraded within 30 cycles. Furthermore, after 200th cycle, the Co3O4 NFs and the Co3O4 NFs@rGO have 924.2 mAh g−1 and 1370.8 mAh g−1 at a current density of 1.0 A g−1, respectively. Figure 5b shows rate capabilities of the Co3O4 NFs and the Co3O4 NFs@rGO between 0.1 and 2.0 A g−1. At all of current densities, the Co3O4 NFs@rGO show much higher capacities than pristine Co3O4 NFs. In addition, the Co3O4 NFs@rGO exhibit high electrochemical performance that shows first discharge capacity of 669 mAh g−1 and 400th discharge capacity of 615 mAh g−1 (cycle retention of 92%) at a high current density of 4.0 A g−1 (Fig. 5c). It is noticeable that a low amount of rGO (~3.5 wt% in composite) is enough to improve electrochemical performance along with 1-D structural effect in a complementary manner. This amount of carbon is overwhelmingly lower than previously reported values (Table S2).
(a) cycle performances of the Co3O4 NFs and the Co3O4 NFs@rGO at a current density of 1.0 A g−1; (b) rate capabilities of the Co3O4 NFs and the Co3O4 NFs@rGO at various current densities between 0.1 and 2.0 A g−1; (c) cycle performance for long-term stability of the Co3O4 NFs@rGO at high current density of 4.0 A g−1.
In Fig. 5, the Co3O4 NFs and the Co3O4 NFs@rGO tend to increase the capacity as the cycle progresses. Such increase in capacity of the Co3O4 NFs may be due to an activated formation of gel-like polymeric film on Co metal surface23. Interestingly, as shown in Fig. 5, the capacity of Co3O4 NFs@rGO has been increased through the graphene wrapping process, and also its capacity is higher than the theoretical capacity of Co3O4 NFs@rGO 
 . These extra capacities can be explained by the interfacial Li+ storage that represents the formation of gel-like polymeric film and the pseudo-capacitive property on Co metal grains. The gel-like polymeric film is the reversible product from the side reaction of electrolyte decomposition on the surface of Co metal grain at low voltage during discharge process, and this film provides extra capacity for lithium storage24,25. In addition, the Co metal grain which came from conversion reaction can allow extra Li+ adsorption site by metallic pseudo-capacitive property26,27,28. In consideration of such extra charge, unlike the pristine Co3O4 NFs, the Co3O4 NFs@rGO provide the fast electron pathway to form negatively charged Co metal grains for effective Li+ adsorption on their surface. Figure 6a shows the differential capacity plots for Co3O4 NFs and Co3O4 NFs@rGO after 20th cycle at a current density of 1.0 A g−1. The broad cathodic peak at around 2.2 V is caused by Li+ insertion to Co3O4NFs, which corresponds to region I in Fig. 6b,c. The peak at 1.23 V is the reduction of Co3O4 to Co metal nanograins (conversion reaction). These peaks are positioned at same potential for both samples, because the plateau appears at the same voltage in Fig. 6b,c. In Fig. 6b,c there are the regions I and II that are related to Li+ insertion and conversion of Co3O4 with Li+ and the region III is relevant to the interfacial Li+ storage ability of active materials occurred in solid-liquid interface with Li+ adsorption29,30,31. Furthermore, the dQ/dV value of Co3O4 NFs@rGO at 1.23 V is twice that of Co3O4 NFs. The broad cathodic peak at around 1.0 V is caused by the interfacial lithium ion storage of electrolyte decomposition on Co metal nanograin and pseudo-capacitive property of metal grains. For the peak at around 1.0 V, the dQ/dV value of Co3O4 NFs@rGO is 1.5 times higher compared with the Co3O4 NFs. Based on these results, the Co3O4 NFs@rGO proceed the same reaction compared to Co3O4 NFs, but the rGO layers promote the degree of Li+ interfacial storage reaction in all cases of Li+ insertion, conversion, and interfacial adsorption.
. These extra capacities can be explained by the interfacial Li+ storage that represents the formation of gel-like polymeric film and the pseudo-capacitive property on Co metal grains. The gel-like polymeric film is the reversible product from the side reaction of electrolyte decomposition on the surface of Co metal grain at low voltage during discharge process, and this film provides extra capacity for lithium storage24,25. In addition, the Co metal grain which came from conversion reaction can allow extra Li+ adsorption site by metallic pseudo-capacitive property26,27,28. In consideration of such extra charge, unlike the pristine Co3O4 NFs, the Co3O4 NFs@rGO provide the fast electron pathway to form negatively charged Co metal grains for effective Li+ adsorption on their surface. Figure 6a shows the differential capacity plots for Co3O4 NFs and Co3O4 NFs@rGO after 20th cycle at a current density of 1.0 A g−1. The broad cathodic peak at around 2.2 V is caused by Li+ insertion to Co3O4NFs, which corresponds to region I in Fig. 6b,c. The peak at 1.23 V is the reduction of Co3O4 to Co metal nanograins (conversion reaction). These peaks are positioned at same potential for both samples, because the plateau appears at the same voltage in Fig. 6b,c. In Fig. 6b,c there are the regions I and II that are related to Li+ insertion and conversion of Co3O4 with Li+ and the region III is relevant to the interfacial Li+ storage ability of active materials occurred in solid-liquid interface with Li+ adsorption29,30,31. Furthermore, the dQ/dV value of Co3O4 NFs@rGO at 1.23 V is twice that of Co3O4 NFs. The broad cathodic peak at around 1.0 V is caused by the interfacial lithium ion storage of electrolyte decomposition on Co metal nanograin and pseudo-capacitive property of metal grains. For the peak at around 1.0 V, the dQ/dV value of Co3O4 NFs@rGO is 1.5 times higher compared with the Co3O4 NFs. Based on these results, the Co3O4 NFs@rGO proceed the same reaction compared to Co3O4 NFs, but the rGO layers promote the degree of Li+ interfacial storage reaction in all cases of Li+ insertion, conversion, and interfacial adsorption.
To further understand the effect of graphene layer wrapping, ex-situ SEM analysis for the Co3O4 NFs@rGO and the Co3O4 NFs were carried out after 100th cycle (Fig. 7a,b). The surface state of the delithiated-Co3O4 NFs@ rGO was observed, indicating that the nanocomposite structure could be intactly preserved with formation of stable SEI layer (Fig. 7a), whereas the SEI layer of the Co3O4 NFs was conspicuously and irregularly generated (Fig. 7b). To further investigate resistance in the nanocomposites, electrochemical impedance spectroscopy (EIS) measurement was conducted. Figure 7c shows the Nyquist plots of the Co3O4 NFs and the Co3O4 NFs@rGO after the 100th cycle and the equivalent model corresponding to the EIS model. The impedance values of each impedance component calculated from the EIS data are represented in Table S3. RE, RSEI, RCT-1, RCT-2 and RP are the ohmic resistance of the cell, the SEI resistance, the charge transfer resistances, and the phase transformation resistance, respectively. In the case of the Co3O4 NFs@rGO, the SEI resistance was reduced by 37.7%, compared to the Co3O4 NFs. Also, the charge transfer resistance of Co3O4 NFs@rGO (18.26 Ω) is much smaller than that of Co3O4 NFs (68.68 Ω). Therefore, it is believed that the low-content rGO layer on Co3O4 NFs is significantly beneficial to enable smaller polarization for LIBs. Here, we proposed the simple reaction models during discharge process for the Co3O4 NFs@rGO and the Co3O4 NFs (Fig. 7d,e). In the case of the Co3O4 NFs surrounded by SEI and super P (carbon additive), electrons may not efficiently transfer between electrode materials (Fig. 7e). Meanwhile, for the Co3O4 NFs@rGO, the 2-D rGO layers not only greatly suppress the formation of insulating SEI layer, but also give facile passage for electron. More importantly, after the lithiation, the rGO can be more effective electron bridge between the discharge products (Co NPs in Li2O matrix) (Fig. 7d). From the point of view of the Li+ interfacial storage, the Co3O4 NFs@rGO is much suitable compared to Co3O4 NFs without such conducting route. These envisioned mechanisms can be supported by higher capacity of the Co3O4 NFs@rGO than that of the Co3O4 NFs in Fig. 5a,b. Based on the data above and our interpretation, it can be thought that combination of 1-D nanostructure and rGO-wrapping is appropriate for desirably designed electrode architecture, strategy of which will be an explicit direction for conversion-based LIBs.
Ex-situ scanning electron microscopy images of (a) the Co3O4 NFs@rGO and (b) the Co3O4 NFs after 100th cycle at a current density of 1.0 A g−1; (c) Nyquist plots of Co3O4 NFs and Co3O4 NFs@rGO after the 100th discharge, presented with fitted curves; The illustration of expected mechanism for electron transfer on (d) Co3O4 NFs@rGO and (e) Co3O4 NFs.
In summary, we designed the ultra-thin rGO layer wrapped 1-D Co3O4 NFs as high performance anode for LIBs, which were simply synthesized via an electrospinning and subsequent self-assembly wrapping of graphene sheets on Co3O4 NFs. 1-D nanostructure of Co3O4 NFs not only overcome the alleviation of pulverization upon cycling, but also provide the fast Li+ diffusion and continuous electric contact. Moreover, the rGO layer, which exists as small percentage of carbon within Co3O4, provides high electric conductivity to Co3O4 NFs for high specific capacity, rendering favorable conversion reaction and Li+ interfacial storage. The Li anode electrode with Co3O4 NFs@rGO delivered a relatively high reversible capacity of 615 mAh g−1 with stable capacity retention of 92% after 400th cycle at the high current density of 4.0 A g−1. Through above results, the reduced graphene oxide sheets wrapped Co3O4 NFs were suggested as high performance anode for LIBs.
Methods
Materials
Cobalt (ii) acetate tetrahydrate (Co(OAc)2·4H2O, Sigma-Aldrich), polyvinylpyrrolidone (PVP, Mw~1,300,000, Sigma-Aldrich), N,N-dimethylformaimde (DMF, 99.8%, Sigma-Aldrich), GO solution (2 ml mg−1, Sigma-Aldrich) and poly(allylamine hydrochloride) (PAH, Mw~900,000, Sigma-Aldrich) were prepared for synthesis of Co3O4 NFs@rGO. Super P (Alfa Aesar), carboxymethylcellulose sodium salt (CMC, Sigma-Aldrich) and poly(acrylic acid) solution (PAA solution, Sigma-Aldrich, 35 wt.% in H2O) were purchased for the electrochemical measurements.
Synthesis of Co3O4 NFs
The Co3O4 NFs were prepared by an electrospinning process and subsequent calcination. 1.5 g of Co(OAc)2·4H2O and 0.5 g of PVP were dissolved in 4 g of DMF. Electrospinning solution was stirred at 500 rpm for 24 h. The solution was transferred into a plastic syringe equipped with stainless needle. The needle gauge was 25 G. The distance between tip of needle and collector was maintained to 15 cm. The applied voltage on the needle tip was DC 15.0 kV. The solution was pulled out with the flow rate of 25 μl min−1. After electrospinning, collected as-spun Co(Ac)2/PVP NFs were heated at 600 °C with heating rate of 5 °C min−1 for 1 h in air atmosphere for removing polymer and crystallizing Co-precursor to Co3O4.
PAH functionalization and graphene wrapping
For graphene wrapping, firstly, 0.25 g PAH was dissolved in 50 ml of distilled water. Then, 0.1 g of the Co3O4 NFs was added in PAH solution. The surface of the Co3O4 NFs was modified to amine group by PAH. After magnetic stirring for 2 h, the PAH-treated Co3O4 NFs were washed three times with distilled water. After that, the PAH-teated Co3O4 NFs were dried at 50 °C for overnight. For preparing graphene solution, GO was dissolved in distilled water up to 66.3 μg ml−1. Sequentially, the PAH-treated Co3O4 NFs were added into the GO solution, and the mixed solution was stirred at 200 rpm for 12 h. After electrostatic assembly between the PAH-treated Co3O4 NFs and the GO, to reduce the GO, 0.5 g of hydrazine monohydrate was added into the solution and stirred at 200 rpm for 2 h. Finally, several washing and drying steps were conducted.
Material characterization
The morphologies of the Co3O4 NFs and the Co3O4 NFs@rGO were confirmed via scanning electron microscopy (SEM, XL-30 SFEG, Philips). The field emission transmission electron microscope (FE-TEM, Tecnai G2 F30 S-Twin, FEI, Netherlands) was used to check the lattice spacing of Co3O4 and the thickness of graphene layer. The energy-dispersive spectrometry (EDS) mapping of scanning transmission electron microscope (STEM) shows the distribution of carbon, cobalt and oxygen atoms on NFs. The x-ray diffraction (XRD) pattern of Co3O4 NFs and Co3O4 NFs@rGO was carried out using X-Ray diffraction-meter (D/MAX-2500, Rigaku, Japan). The range of measured X-ray diffraction angle was 2θ = 20–70° using Cu-Kα (λ = 1.54 Å) radiation. The amount of carbon component of the Co3O4 NFs@rGO was analyzed by element analyzer (FLASH 2000 series, Thermo Scientific). To investigate the graphene sheets, Raman spectroscopy (Aramis, Horiba Jobin Yvon, France) was used.
Electrochemical measurements
To measure electrochemical performance, active materials were mixed with Super P and binders. The binders were used with CMC solution and PAA solution. The ratio of active material (Co3O4 NFs or Co3O4 NFs@rGO), super P, CMC solution and PAA solution was 75:15:5:5 in weight. Mixed slurry was coated on Cu-foil with a thickness of 90 μm. Sequentially, the coated Cu-foil was dried at 50 °C for 20 min for removing solvents. For evaporation of moisture and activation of binder, the coated Cu-foil was dried in vacuum oven at 150 °C for 2 h. The obtained anode was assembled to CR2032 coin-type cell in an argon gas filled glove box. The lithium metal was used as counter electrode. The other components of coin cell were Celgard® 2400 as separator, 1.3 M of LiPF6 dissolved in EC/DEC = 3/7 (v/v) +10.0 wt% FEC as electrolyte where EC is ethylene carbonate, DEC is diethyl carbonate, and FEC is fluoroethylene carbonate. Loading mass of active material on Cu-foil was 0.88 ± 0.01 mg cm−2. Current-Voltage curve was measured at Wonatech WBCS3000 in the voltage window of 0.01–3.0 V and a scan rate of 0.5 mV s−1. Galvanostatic charge/discharge process was carried out by Maccor series 4000 in the voltage window of 0.01–3.0 V. Electrochemical impedance spectroscopy (EIS) was performed to investigate the impedance change after 100th cycle with one channel potentiostat (ZIVE SP1 potentiostat, Wonatech, Korea) by applying a sine wave of 10 mV in the frequency range of 105–0.01 Hz.
Additional Information
How to cite this article: Cho, S.-H. et al. Rational Design of 1-D Co3O4 Nanofibers@Low content Graphene Composite Anode for High Performance Li-Ion Batteries. Sci. Rep. 7, 45105; doi: 10.1038/srep45105 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2008).
Goodenough, J. B. & Park, K. S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 135, 1167–1176 (2013).
Palacín, M. R. Recent advances in rechargeable battery materials: a chemist’s perspective. Chem. Soc. Rev. 38, 2565–2575 (2009).
Tarascon, J.-M., Poizot, P., Laruelle, S., Grugeon, S. & Dupont, L. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000).
Reddy, M. V., Rao, G. V. S. & Chowdari, B. V. R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 113, 5364–5457 (2013).
Cabana, J., Monconduit, L., Larcher, D. & Palacín, M. R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, 170–192 (2010).
Ryu, W.-H., Shin, J., Jung, J.-W. & Kim, I.-D. Cobalt(ii) monoxide nanoparticles embedded in porous carbon nanofibers as a highly reversible conversion reaction anode for Li-ion batteries. J. Mater. Chem. A 1, 3239–3243 (2013).
Ren, L. et al. 3D hierarchical porous graphene aerogel with tunable meso-pores on graphene nanosheets for high-performance energy storage. Sci. Rep. 5, 14229, 10.1038/srep14229 (2015).
Park, J. et al. Three-dimensional aligned mesoporous carbon nanotubes filled with Co3O4 nanoparticles for Li-ion battery anode applications. Electrochim. Acta 105, 110–114 (2013).
Yao, X. et al. Synergistic Effect of Mesoporous Co3O4 Nanowires Confined by N-Doped Graphene Aerogel for Enhanced Lithium Storage. Small 28, 3849–3860 (2016).
Li, L. et al. Co3O4 mesoporous nanostructures@graphene membrane as an integrated anode for long-life lithium-ion batteries. J. Power Sources 255, 52–58 (2014).
Li, B. et al. Co3O4@graphene composites as anode materials for high-performance lithium ion batteries. Inorg. Chem. 50, 1628–1632 (2011).
Wu, Z. S. et al. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 4, 3187–3194 (2010).
Stankovich, S. et al. Graphene-based composite materials. Nature 442, 282–286 (2006).
Raccichini, R., Varzi, A., Passerini, S. & Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 14, 271–9 (2015).
Jung, J. W., Ryu, W. H., Shin, J., Park, K. & Kim, I. D. Glassy Metal Alloy Nanofiber Anodes Employing Graphene Wrapping Layer: Toward Ultralong-Cycle-Life Lithium-Ion Batteries. ACS Nano 9, 6717–6727 (2015).
Shin, J., Park, K., Ryu, W.-H., Jung, J.-W. & Kim, I.-D. Graphene wrapping as a protective clamping layer anchored to carbon nanofibers encapsulating Si nanoparticles for a Li-ion battery anode. Nanoscale 6, 12718–12726 (2014).
Zhou, W. et al. A general strategy toward graphene@metal oxide core–shell nanostructures for high-performance lithium storage. Energy Environ. Sci. 4, 4954–4961 (2011).
Jiang, J. & Li, L. Synthesis of sphere-like Co3O4 nanocrystals via a simple polyol route. Mater. Lett. 61, 4894–4896 (2007).
Claramunt, S. et al. The importance of interbands on the interpretation of the raman spectrum of graphene oxide. J. Phys. Chem. C 119, 10123–10129 (2015).
Huang, G. et al. Metal Organic Frameworks Route to in situ Insertion of Multiwalled Carbon Nanotubes in Co3O4 Polyhedra as Anode Materials for Lithium-Ion Batteries. ACS Nano 9, 1592–1599 (2015).
Geng, H. et al. Porous cubes constructed by cobalt oxide nanocrystals with graphene sheet coatings for enhanced lithium storage properties. Nanoscale 8, 7688–7694 (2016).
Grugeon, S., Laruelle, S., Dupont, L. & Tarascon, J.-M. An update on the reactivity of nanoparticles co-based compounds towards Li. Solid State Sci. 5, 895–904 (2003).
Laruelle, S. et al. On the Origin of the Extra Electrochemical Capacity Displayed by MO/Li Cells at Low Potential. J. Electrochem. Soc. 149, A627–A634 (2002).
Lee, K. T. & Cho, J. Roles of nanosize in lithium reactive nanomaterials for lithium ion batteries. Nano Today 6, 28–41 (2011).
Ranganath, S. B., Hassan, A. S., Ramachandran, B. R. & Wick, C. D. Role of Metal-Lithium Oxide Interfaces in the Extra Lithium Capacity of Metal Oxide Lithium-Ion Battery Anode Materials. J. Electrochem. Soc. 163, A2172–A2178 (2016).
Jamnik, J. & Maier, J. Nanocrystallinity effects in lithium battery materials. Phys. Chem. Chem. Phys. 5, 5215–5220 (2003).
Maier, J. Size effects on mass transport and storage in lithium batteries. J. Power Sources 174, 569–574 (2007).
Kim, H.-S., Cook, J. B., Tolbert, S. H. & Dunn, B. The Development of Pseudocapacitive Properties in Nanosized-MoO2 . J. Electrochem. Soc. 162, A5083–A5090 (2015).
Chen, C. et al. Integrated Intercalation-Based and Interfacial Sodium Storage in Graphene-Wrapped Porous Li4Ti5O12 Nanofibers Composite Aerogel. Adv. Energy Mater. 6, 2–9 (2016).
Yu, S., Jung, J.-W. & Kim, I.-D. Single layers of WS2 nanoplates embedded in nitrogen-doped carbon nanofibers as anode materials for lithium-ion batteries. Nanoscale 7, 11945–11950 (2015).
Acknowledgements
This work was supported by Korea CCS R&D Center(KCRC) grant funded by the Korea government (Ministryof Science, ICT & Future Planning) (No. NRF-2014M1A8A1049303), Wearable Platform Materials Technology Center (WMC) funded by a National Research Foundation of Korea (NRF) Grant of the Korean Government (MSIP) (NRF-2016R1A5A1009926), End-Run grant from KAIST funded by the Korea government in 2016 (Ministry of Science, ICT & Future Planning) (N011160058), and Korea Institute of Machinery & Materials (KIMM) and the National Research Council of Science and Technology (NST), Republic of Korea.
Author information
Authors and Affiliations
Contributions
S.H.C. and J.W.J. contributed to implement the concept. S.H.C. performed nearly all parts of the experimental system and carried out the measurements and characterizations, and C.H.K. assisted with experimental system and J.W.J. provide research advice. S.H.C., J.W.J. and C.H.K. wrote the text of the entire manuscript and prepared all figures. I.D.K. supervised the project and led the effort to completion. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cho, SH., Jung, JW., Kim, C. et al. Rational Design of 1-D Co3O4 Nanofibers@Low content Graphene Composite Anode for High Performance Li-Ion Batteries. Sci Rep 7, 45105 (2017). https://doi.org/10.1038/srep45105
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45105
This article is cited by
-
Enhanced Electrochemical Performance of Low-Content Graphene Oxide in Porous Co3O4 Microsheets for Dual Applications of Lithium-Ion Battery Anode and Lithium-Ion Capacitor
Journal of Electronic Materials (2024)
-
Preventive agents for neurodegenerative diseases from resin of Dracaena cochinchinensis attenuate LPS-induced microglia over-activation
Journal of Natural Medicines (2019)
-
High performance Co3O4/Li2TiO3 composite hollow nanofibers as anode material for Li-ion batteries
Journal of Materials Science: Materials in Electronics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.