Abstract
Prostacyclin, also termed as prostaglandin I2 (PGI2), evokes contraction in vessels with limited expression of the prostacyclin receptor. Although the thromboxane-prostanoid receptor (TP) is proposed to mediate such a response of PGI2, other unknown receptor(s) might also be involved. TP knockout (TP−/−) mice were thus designed and used to test the hypothesis. Vessels, which normally show contraction to PGI2, were isolated for functional and biochemical analyses. Here, we showed that the contractile response evoked by PGI2 was indeed only partially abolished in the abdominal aorta of TP−/− mice. Interestingly, further antagonizing the E-type prostaglandin receptor EP3 removed the remaining contractile activity, resulting in relaxation evoked by PGI2 in such vessels of TP−/− mice. These results suggest that EP3 along with TP contributes to vasoconstrictor responses evoked by PGI2, and hence imply a novel mechanism for endothelial cyclooxygenase metabolites (which consist mainly of PGI2) in regulating vascular functions.
Similar content being viewed by others
Introduction
Cyclooxygenase (COX), which exists mainly as COX-1 and -2 isoforms, mediates the metabolism of arachidonic acid (AA) to produce vasoactive prostanoids1,2,3,4. Among them, thromboxane (Tx) A2 and prostacyclin (prostaglandin I2; PGI2) have been considered to represent two opposing regulatory mechanisms in the cardiovascular system. TxA2 is mainly produced in platelets and it acts on the thromboxane-prostanoid receptor (TP) to mediate vasoconstriction and platelet-aggregation. In contrast, PGI2 is mainly synthesized in the vascular endothelium and is proposed to activate the PGI2 receptor (IP) that mediates vasodilatation and opposes the effects of TP. An imbalance between the effects derived from endothelial PGI2 and those of platelet-produced TxA2 is though to result in the development of cardiovascular disorders, such as hypertension1,2,3,4,5,6.
On the other hand, in some vascular beds (including certain human vessels), PGI2 or endothelial COX metabolites (which consist mainly of PGI2) evoke contraction via the activation of TP7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24. Studies have further revealed that vasomotor reactions to PGI2 are modulated by both IP and TP; hence a vasoconstrictor response evoked by PGI2 or endothelial COX metabolites reflects limited expression or function of IP, which leads to the uncovering of vasoconstrictor activity derived from concurrently activated TP8,20,21,22,25,26,27,28,29,30,31,32. However, in some vessels, such as mouse abdominal aorta where IP is expressed (although to a lesser extent as compared to vessels showing dilation to the agonist), PGI2 does not evoke relaxation even after TP blockade28,30. Also, in some vascular beds, the contraction evoked by PGI2 or endothelial COX metabolites is less sensitive to TP blockade11,22. We propose that in addition to TP, other receptor(s) can also be involved in PGI2-evoked vasoconstrictor activity. However, the existence of such a mechanism or the identity of the additional receptor(s) remains to be elucidated. In addition, the involvement of TP in the vasoconstrictor activity of PGI2 has been primarily based on results with pharmacological blockade, which also inhibits contractions evoked by other PGs or AA-related metabolites8,33,34. Thus, it would also be of interest to evaluate the precise role of TP in PGI2-evoked vasoconstrictor responses with genetic manipulation.
To resolve the above issues, in this study we generated a strain of TP−/− mice on a C57BL/6 background. Aortas, carotid and/or renal arteries, where PGI2 evokes vasoconstrictor response under normal conditions26,28,30,35, were isolated for biochemical and/or functional analyses.
Results
Mutation in TP−/− mice and phenotype
As shown in Fig. 1a, sequencing of TP DNA PCR products revealed that as compared with that of wild-type (WT) mice, exon 3 of the TP locus in TP−/− mice has a 22 bp fragment deletion (CTG GGG GCC TGC TTT CGC CCG G) in the coding area, which was 18 bp after the start codon (NCBI Reference Sequence: NM_009325.3). This resulted in a frame-shift in TP mRNA transcript and a premature termination of protein translation (only 7 amino acids were coded before the appearance of a stop codon (TGA) in TP−/− mice; Fig. 1a, bottom right). Indeed, RT-PCR revealed that un-mutated TP mRNAs, which were abundant in WT aortas, were not detected in the TP−/− counterparts (Fig. 1b). Also, compared to WT controls, TP−/− mice had an elongated bleeding time (Fig. 1c). However, these mice appear normal, and show no overt abnormality in mean arterial blood pressure (MAP; 92.3 ± 3.3 vs. 95.0 ± 2.8 mmHg in WT mice, n = 5 for each; P > 0.05) or in reproduction.
(a) mutation in the TP locus. Top: WT DNA sequencing showing the surrounding sequences and the fragment to be deleted (underlined) in TP−/− mice. Bottom left: sequencing of mutated DNA showing the deletion of 22 bp DNA fragment in TP−/− mice. The bar separates the upper and down stream sequences of the deleted fragment. Bottom right: partial sequences of TP mRNA transcripts or those of proteins to be translated in WT (upper) and TP−/− mice (lower). (b) RT-PCR showing the expressions of un-mutated mRNAs in WT and TP−/− mouse aortas. Bands were visualized with a SYBR Safe DNA gel stain (Thermo Scientific) and the image was captured by an electrophoresis imaging cabinet (Universal Hood II; Bio-rad, Hercules, CA, USA). M: 100 bp ladder size marker (Thermo Scientific). (c) bleeding time in TP−/− and WT mice. Values are expressed as mean ± SEM; n = 5; **P < 0.01.
Effect of TP−/− on contractions evoked by PGI2 and other prostanoids
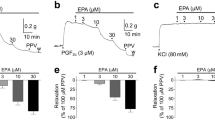
Abdominal aortas were then isolated for functional analyses. Vessels were treated with the NO synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (L-NAME; 1 mM). In WT vessels, the TP agonist U46619 evoked potent contraction as noted previously28; however, in TP−/− mice, U46619 did not evoke any response (Fig. 2a). Interestingly, not only the contraction evoked by PGI2 (Fig. 2b), but that to PGF2α (Fig. 2b), PGE2 (Fig. 2c), or PGD2 (Fig. 2d) was also diminished or largely removed in TP−/− vessels. At the same time, the contraction to low concentrations (0.1–0.3 μM) of PGE2 remained intact in TP−/− vessels, and this contraction was abolished by the E-type prostaglandin receptor EP3 antagonist L798106 (1 μM), but not by the TP antagonist SQ29548 (10 μM; Fig. 2c). In addition, L798106 abolished the remaining contraction evoked by PGD2 in TP−/− vessels (Fig. 2d).
(a,b) comparison of contractions evoked by the TP agonist U46619 (a), and PGI2 or PGF2α (b) in WT and TP−/− vessels. (c,d) contraction to PGE2 (c) or PGD2 (d) in WT or TP−/− mice and that of TP−/− vessels treated with the TP antagonist SQ29548 (10 μM; +SQ) or the EP3 antagonist L798106 (1 μM; +L). Values are expressed as mean ± SEM; n = 5 for each. **P < 0.01 vs. the value of WT mice; ++P < 0.01 vs. TP−/− mice.
PGI2-induced response in TP−/− abdominal aortas precontracted with PE
Next, we determined whether PGI2-evoked contractile response in TP−/− vessels was masked by a dilator effect of the agonist. To this end, L-NAME-treated or endothelium-denuded abdominal aorta were precontracted with phenylephrine (PE; 2 μM), under which the vasoconstrictor response to an agonist is more readily detectable compared to baseline conditions28. Under either condition, PGI2 (1 μM) evoked an increase of force on PE-induced contraction, which was however reversed by the EP3 antagonist L798106 (1 μM) into relaxation (Fig. 3a,b). In contrast, the EP1 antagonist SC19220 (10 μM) had no effect (Fig. 3a). Also, L798106, but not SC19220 inhibited a similar response evoked by PGE2 (0.1 μM), although no relaxation was observed (Fig. 3c,d). Meanwhile, forces of PE-evoked contractions were found to be comparable among vessel groups that had been treated with L-NAME (Fig. 3a,c bottom panels).
(a) summaries (n = 5 for each) of responses (top) to 1 μM PGI2 and forces of PE-evoked contractions (pre-force; bottom) in control L-NAME-treated TP−/− vessels or those additionally with the EP3 antagonist L798106 (1 μM; +L) or the EP1 antagonist SC19220 (10 μM; +SC). (b) representative traces with summarized values showing the control response to PGI2 in endothelium-denuded TP−/− vessels [TP−/−/EC (−)] or that with L798106 (+L). *P < 0.05. (c) summaries (n = 5 for each) of responses (top) to 0.1 μM PGE2 and forces of PE-evoked contractions (bottom) as in (a). **P < 0.01 vs. control value of TP−/− vessels. In (a–c), *P < 0.05 or **P < 0.01 vs. control value of TP−/− vessels. Data are expressed as mean ± SEM. (d) representative traces showing the response to PGE2 in L-NAME-treated TP−/− vessels (TP−/−) or that obtained with L798106 (+L).
Effect of EP3 antagonism on ACh-evoked contraction in TP−/− or TP-inhibited abdominal aortas
In the mouse abdominal aorta, the muscarinic agonist acetylcholine (ACh) activates endothelial COX to mainly produce PGI2 and evoke contraction under NOS-inhibited conditions8,28,35. Therefore, responses evoked by the maximal concentration of ACh were examined in L-NAME-treated TP−/− or TP-inhibited abdominal aortas8,13,14,15.
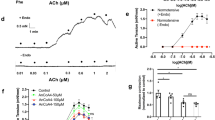
As compared to that of WT controls, the contraction evoked by ACh (10 μM) in TP−/− vessels was indeed mostly abolished; however, a minor contraction could still be produced (Fig. 4a). Moreover, in such-treated TP−/− vessels precontracted with PE (2 μM), ACh evoked relaxation, which was blunted by a biphasic force sensitive to the non-selective COX inhibitor indomethacin. Interestingly, the EP3 antagonist L798106 also abolished the biphasic force, resulting in relaxation, which was to a greater extent than that obtained with indomethacin (Fig. 4b,c). In addition, such an enhancement of relaxation resulting from L798106 was removed by the IP antagonist CAY10441 (1 μM; Fig. 4c).
(a) representative traces with summarized values showing responses to ACh (10 μM) under baseline conditions in WT (top) and TP−/− (bottom) vessels. P < 0.01 vs. WT vessels (b,c) representative traces (b) and/or summaries of time-courses of responses to ACh (c top) along with forces of PE-evoked contractions (pre-force; c bottom) in precontracted TP−/− vessels or those obtained with the non-selective COX inhibitor indomethacin (10 μM; +IND), with the EP3 antagonist L798106 (1 μM; +L) or with both L789106 and the IP antagonist CAY10441 (1 μM; +L/CAY). (d) time-courses of responses to ACh (top) and forces of PE-evoked contractions (pre-force; bottom) in precontracted WT vessels in the presence of the TP antagonist SQ29548 (WT/SQ) or in those additionally treated with L798106 (1 μM; +L) or both L798106 and indomethacin (+L/IND). In (c and d) **P < 0.01 vs. TP−/− or WT/SQ; ++P < 0.01 vs. TP−/−/L or +L. Data were expressed as mean ± SEM (n = 5 for each).
Likewise, in similar PE-precontracted WT vessels where the agonist usually evokes an increase of force28, treatment with the TP antagonist SQ29548 (10 μM) caused relaxation that was also blunted by a biphasic force in response to ACh. Again, the EP3 antagonist L798106 (1 μM) abolished the force, resulting in an enhanced relaxation that was reduced by indomethacin (Fig. 4d top). No significant difference was found among forces of PE-evoked contractions in TP−/− or WT vessels (Fig. 4c,d bottom panels).
Effect of TP−/− on endothelial COX products and expressions of PG receptors
The production of PGI2 and some other prostanoids evoked by ACh in TP−/− and WT aortas was then examined. As shown in Fig. 5a, in WT and TP−/− aortas ACh evoked an increase in the production of the PGI2 metabolite 6-keto-PGF1α, which was comparable between the two mouse strains. Also, an increase of PGE2 was obtained with ACh, although levels were ~10-fold lower than those of 6-keto-PGF1α (Fig. 5b). No significant difference was found in amounts of PGE2 between TP−/− and WT vessels (Fig. 5b). In contrast, the TxA2 metabolite TxB2 was not increased by ACh in vessels from either mouse strain (Fig. 5c), similar to results reported previously34,36.
(a–c) summaries of the PGI2 metabolite 6-keto-PGF1α (a), PGE2 (b), and the TxA2 metabolite TxB2 (c) in TP−/− and WT aortas under the basal and ACh (10 μM)-stimulated conditions. (d) real-time PCR detection of IP, EP3 and FP mRNAs in TP−/− and WT aortas. The level of mRNAs was normalized by that of β-actin with the average value of WT assumed as 1.0. *P < 0.05 and **P < 0.01; NS: not significant. Data are expressed as mean ± SEM (n = 6 for each).
The expressions of IP, EP3 and the PGF2α receptor (FP) mRNAs were also determined. As shown by real-time PCR, the level of IP, EP3 or FP mRNAs normalized by that of β-actin in TP−/− aortas was comparable with that of WT counterparts (Fig. 5d).
Effect of EP3 antagonism on varied vasoconstrictor responses in WT vessels
The effect of EP3 antagonism was further determined in L-NAME-treated WT vessels. As shown in Fig. 6a, in WT abdominal aortas the EP3 antagonist L798106 (1 μM) diminished the contraction evoked by PGI2. Also, in such vessels precontracted with PE (2 μM), PGI2 (1 μM) or the COX substrate AA (3 μM), whose response is sensitive to TP antagonism under baseline conditions35, evoked an increase of force in the presence of the TP antagonist SQ29548 (10 μM) but a relaxation that was sensitive to the IP antagonist CAY10441 (1 μM) when both SQ29548 and L798106 were present (Fig. 6b). No significant difference was found among forces of PE-evoked contractions (Fig. 6b bottom).
(a) control (CTL) responses evoked by PGI2 under baseline conditions in the abdominal aorta (AAo), and that obtained with the EP3 antagonist L798106 (1 μM; +L) (b) responses (top) to PGI2 (1 μM) or AA (3 μM) and forces of PE-evoked contractions (pre-force; bottom) in pre-contracted AAo treated with the TP antagonist SQ29548 (10 μM; WT/SQ) or those additionally with L798106 (1 μM; +SQ/L) or both L789106 and the IP antagonist CAY10441 (1 μM; +SQ/L/CAY). **or ++P < 0.01 vs. WT/SQ or +SQ/L, respectively. (c) effect of L789106 (1 μM; +L) or SQ29548 (10 μM; +SQ) on the contraction to PGE2 (10 μM; PGE2) or ACh (10 μM) in AAo. (d) effect of L789106 (1 μM; +L) on contraction to 1 or 10 μM PGI2 in carotid (CA) and renal arteries (CA), respectively. In (a,c and d) *P < 0.05, and **P < 0.01 vs. WT control (WT/CTL). Data are expressed as mean ± SEM (n = 5 for each).
Interestingly, in WT abdominal aortas, L798106, which drastically diminished the contraction evoked by ACh, only slightly reduced that evoked by a sub-maximal concentration of PGE2 (10 μM; Fig. 6c). However, this contraction to PGE2 was very sensitive to the TP antagonist SQ29548 (Fig. 6c). In addition, L798106 diminished the contraction evoked by 1 or 10 μM PGI2 in carotid and renal arteries (Fig. 6d).
Discussion
In this study we show that in NOS-inhibited WT mouse abdominal aortas PGI2- or ACh (which activates endothelial COX to mainly produce PGI2) evokes contraction that is diminished in TP−/− counterparts. More importantly, in TP−/− vessels or TP-inhibited vessels of WT mice, antagonizing EP3 abolishes the remaining vasoconstrictor responses to these agonists, resulting in relaxations sensitive to IP and/or COX blockade. EP3 antagonism also diminishes the contraction evoked by PGI2 and/or ACh in NOS-inhibited WT abdominal aorta, carotid and renal arteries. These results not only demonstrate that TP contributes only partially to the contraction evoked by PGI2, but also suggest that EP3 has an important involvement in the response.
The deletion of TP in TP−/− mice was confirmed by DNA sequencing, mRNA analyses and an elongation of bleeding time37. Indeed, abdominal aortas from these mice (which posses a normal MAP, as reported previously38) lost contraction in response to the TxA2 analogue and TP agonist U46619 even under NOS-inhibited conditions. Notably, in such vessels, not only the contraction to PGI2, but also that to PGF2α, PGE2, or PGD2 was diminished. In contrast, levels of IP, FP and EP3 mRNAs were similar between TP−/− and WT vessels, arguing against that the above reduced PG responses resulted from altered expressions of receptors. Thus, TP, which appears able to be activated by all vasoactive prostanoids that are structurally similar4, mediates PGI2’s contractile activity. Due to practical reasons, we were unable to clearly detect IP, EP3, and FP at the protein level; however, our above idea concurs with results in WT mice and some other species obtained here or previously with pharmacological blockade8,28,34. At the same time, responses evoked by low concentrations (0.1–0.3 μM) of PGE2 in TP−/− vessels reveal a functional role of EP3 unaffected by the TP antagonist used.
Interestingly, we further noted that antagonizing EP3 abolished the remaining contraction, resulting in relaxation in response to PGI2 in either NOS-inhibited or endothelium-denuded TP−/− abdominal aortas. This suggests that EP3, which exists in medial smooth muscles in a manner similar to that of IP and TP, mediates PGI2’s contractile response, although its effect is largely offset by IP when TP is absent. In support of the idea, in NOS-inhibited, TP-antagonized WT vessels a relaxation sensitive to IP antagonism was also evoked by PGI2 following EP3 blockade. Moreover, after EP3 antagonism the contraction to PGI2 was minimal. This suggests that the part of EP3-mediated activity could be only slightly smaller than that of TP, which alone could also be largely masked by IP-mediated effect. Therefore, the robust contractile response to PGI2 in WT vessels reflects activities from both TP and EP3 overcoming the effect of concomitantly activated IP. In contrast, EP1 (another vasoconstrictor PGE2 receptor), EP2 and EP4 (dilator PGE2 receptors) do not appear to have a role in the vessels studied, as suggested by the lack of effect caused by antagonism or the absence of relaxation to PGE2 in TP−/− vessels even after EP3 is antagonized39,40.
Also, the above effects of TP−/−, TP and/or EP3 antagonism under NOS-inhibited conditions were similarly obtained in responses evoked by ACh or AA, which stimulates endothelial COX to mainly produce PGI228,35. As seen from EIA measurements, the profile of COX-derived products in aortas was unaltered by TP−/−. Thus, the mechanism for the contraction evoked by endothelial COX metabolites produced in situ is similar to that of PGI2. Due to an endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation concomitantly activated28,41, the effect of EP3 antagonism on the response evoked by ACh in NOS-inhibited, TP−/− or TP-inhibited vessels was reflected by abolition of the contractile activity blunting EDHF-mediated relaxation and a relaxation that is sensitive to IP or COX blockade, but adds to that of EDHF. One must note that the EDHF activity in the vessel does not originate from non-COX AA metabolites, as we put forward previously35. Indeed, this point is also supported here by the lack of IP-independent relaxation to AA after TP and EP3 were both antagonized.
Previously, the contractile role of EP3 in PGE2-evoked vasoresponse was established in vessels of mice as well as those of humans32,42. In the present study, our results further suggest an intimate link between EP3 and the contractile activity evoked by PGI2. Notably, PGD2 might also act on EP3 to mediate a minor contraction, as revealed by functional studies of its response in TP−/− vessels. These results could again be possibly due to a structural similarity among PGs. In support of this, iloprost, a PGI2 analog, also activates EP343. Moreover, EP3 antagonism exerts a greater inhibitory effect on PGI2-evoked contraction than on that of PGE2 (whose response via EP3 peaks at 0.3 μM, as seen from its response in TP−/− vessels). This implies not only that the EP3 antagonist used has limited if any, unintended effects on TP, but also that PGI2, although it might have a lower potency, is more effective on EP3 than PGE2, underscoring the importance of PGI2 in EP3-mediated vasoconstrictor activities. Besides, the effects of its antagonism among WT vessels studied further indicate that the involvement of EP3 in PGI2’s vasoconstrictor activity is not limited to any specific vascular bed.
Therefore, our above results make important amendments to previous proposals on the mechanisms of PGI2 or endothelial COX metabolites (which consist mainly of PGI2) in mediating vasoconstrictor responses20,44. It should be noted that the contraction to PGI2 only exists in vessels with limited expression of IP44. This is also true in the abdominal aorta examined here where we previously showed that IP expression is lower than in mesenteric arteries (where 0.3 μM PGI2 almost completely relaxes 2 μM PE-evoked contraction)28. A reason for this could be that PGI2 (the prototype IP agonist) is more potent on IP than TP and/or EP3, leading to PGI2 being more likely to evoke relaxation than to cause contraction. Indeed, this idea explains why PGI2 has been recognized as a potent vasodilator in many vascular beds and used clinically as an effective therapy for pulmonary hypertension or peripheral arterial diseases2,6,45,46.
On the other hand, it must also be emphasized that the minimal concentration of PGI2 required to initiate vasoconstrictor activity could be 0.003–0.03 μM (under precontracted conditions), far below the amount (1 ng/mg 6-keto-PGF1α can be translated into 2.7 μmol PGI2 per kg of vessel) released by agonists, such as ACh, or similar to that of it (PGI2) to evoke relaxation in vessels, such as mouse mesenteric arteries even after TP is antagonized28,30,44. Also, PGI2-mediated contraction or endothelial COX-derived vasoconstrictor activity has been found in many vessels across species (including those of humans), of which some are small or resistance arteries23,24,29,34,47. Moreover, PGI2’s contractile activity exists in vessels that show a dilator response to the agonist27,32. As a result, although PGI2 may cause a hypotensive effect in general, concurrent activities via TP and/or EP3 can negate some of its beneficial effects via IP, especially on local vascular pathology under disease conditions22,26,36. For this reason, antagonizing TP and/or EP3 might be needed for an optimal therapeutic effect obtained with PGI2 or its analogues under clinical conditions.
In contrast to our findings, EP1 antagonism has also been suggested to diminish PGI2-evoked contraction48. However, the EP1 antagonist used was also a partial antagonist of TP, which was deleted in the vessels we studied49, not to mention the variation that might exist among species or vascular beds. Also, the COX inhibitor indomethacin may cause off-target effects50,51; however, this agent has been shown not to alter ACh responses in similar vessels of COX-1−/− mice28. Indeed, IP blockade inhibited the relaxation in a manner similar to that of indomethacin. Thus, the effect of indomethacin noted here can be considered to result mainly from COX inhibition. However, the precise structural properties responsible for different PGs to activate the same receptor or for one PG to act on different receptors still require further investigation. Also, reasons for one PG to evoke contraction mainly through receptors other than its own, e.g. FP of PGF2α need to be resolved, given that contractions evoked by endothelial COX metabolites can result from non-PGI2 products, including PGF2α19,20,34,52,53.
In summary, our results demonstrate that TP, which appears able to be activated by all vasoactive prostanoids, only partially mediates PGI2’s vasoconstrictor activity. Interestingly, our data further suggest that PGI2 also effectively activates EP3, whose activity along with that of TP can overcome the dilator effect of concomitantly activated IP to produce a robust vasoconstrictor response, and hence imply a novel mechanism for endothelial COX metabolites (which consist mainly of PGI2) in regulating vascular functions.
Material and Methods
Chemicals and solution
L-NAME, ACh, PE, AA, and the non-selective COX inhibitor indomethacin were purchased from Sigma (St Louis, MO, USA). The TP agonist U46619, PGI2, PGF2α, PGE2, and PGD2, the TP antagonist SQ29548, the IP antagonist CAY10441, the EP3 antagonist L798106, and the EP1 antagonist, SC19220 were bought from Cayman Chemical (Ann Arbor, MI, USA). The composition of physiological salt solution (PSS; pH 7.4 with 95%O2–5% CO2) was as follows (in mM): NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25, and D-glucose 11.5. The 60 mM K+ -PSS (K+) was prepared by replacing an equal molar of NaCl with KCl.
L-NAME, PE, AA, and ACh were dissolved in distilled water (purged with N2 for dissolving AA), while PGI2 was dissolved in carbonate buffer (50 mM, pH 10.0). PGF2α, PGE2, PGD2, CAY10441, SQ29548, L798106, and indomethacin were dissolved in dimethyl sulfoxide (DMSO). The final ratio of a solvent (distilled water, carbonate buffer, or DMSO) to working PSS was 0.5/1,000, which doesn’t alter the final pH value of the working buffer (pH 7.4). The concentration of an inhibitor or antagonist used was based on previous reports, which would selectively inhibit the effect of its intended target27,54,55.
Animals and tissue preparation
All procedures were in conformance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996), and approved by The Institutional Animal Research and Use Committee of Shantou University.
The breeder male and female TP−/− mice (C57BL/5 background) were custom produced by Viewsolid Biotech (Beijing, China), using transcription activator-like effector nuclease technology that targets the TP gene to result in deletion of a 22 bp DNA fragment, 18 bp after the start codon in exon 3 of the TP locus56. WT mice (C57BL/6) were purchased from Vital River (Beijing, China). Male WT mice or TP−/− progenies of 8–12 wk of age were used for experiments. Mice were killed by CO2 inhalation. For in vitro functional and biochemical analyses, aortas, carotid and/or renal arteries were isolated and dissected free of adherent tissues with the help of a binocular microscope.
DNA sequencing
The gene mutation in TP−/− mice was verified by sequencing PCR products (the sense and anti-sense PCR primers were 5′-GAA AGG GTA TTT TGT TCC TGA GGC-3′ and 5′-GCT ACC CCC ATG AAG TAG CAC AGG-3′, respectively) of DNA isolated from tail biopsies and performed by Sangon Biotech (Shanghai, China).
RT-PCR and real-time PCR
The preparation of total RNA from whole sections of mouse aortas and RT reactions were performed as described elsewhere previously28. First-strand cDNA was synthesized using total RNA (250 ng) and oligo(dT)15 primers (TaKaRa; Dalian, China).
TP mRNA transcripts were detected with RT-PCR. Primers for TP were 5′-CTG GGG GCC TGC TTT CGC CCG G-3′ (sense; using the deleted fragment) and 5′–GTC AGG AAG CAC CAA GAG CC-3′ (antisense), while those for β-actin (internal control) were as described previously28. The expected sizes of the RT-PCR products were 530 bp for TP and 300 bp for β-actin.
Expressions of IP, EP3 or FP mRNAs were analyzed by real-time PCR. Primers for EP3 or FP were as follows: 5′-CAG AAT CAC CAC GGA GAC G-3′ (EP3 sense) and 5′-TGC ATT GCT CAA CCG ACA T-3′ (EP3 antisense), and 5′-TCC TTG GAC ACC GAG ATT AT-3′ (FP sense) and 5′–GCA ACG ACT GGC AAG TTT AT-3′ (FP antisense). Those for IP and β-actin (internal control) were described previously28. Real-time PCR was performed using a SYBR PrimScript RT-PCR kit (Thermo Scientific, Carlsbad, CA, USA).
Blood pressure measurement
In some experiments, blood pressure in mice (body weight of 26–30 g) was measured using a computerized noninvasive blood pressure system (Kent Scientific Corporation, Torrington, CT, USA). Mice were accustomed to tail-cuff blood pressure measurements for 3 consecutive days, and then blood pressure was measured on the 4th day. MAP taken from the averaged value of three measurements was used for analysis.
Tail bleeding time assay
To evaluate in vivo bleeding time, WT and TP−/− tails (age, tail size and length matched) were cut 2 mm from the tips, and wounds were then gently wiped with sterilized filter paper every 30 s, until no more blood was visible. The bleeding time was calculated from the ending of cutting to the time when no more blood would be seen on paper.
Analysis of vascular function
Abdominal sections of aortas and main stems of carotid or renal arteries were cut into 1 mm rings. Analysis of vascular function was performed with isometric force measurement as described elsewhere previously28,30. For some experiments, the endothelium was denuded by rotating vessel rings around two wires with passive tension kept at 100 mg (endothelial removal was confirmed by absence of relaxation to 10 μM ACh at the end of experiment).
To remove the influence of endothelial NO, vessels were treated with the NOS inhibitor L-NAME (1 mM), under which the response of arteries appears similar to that of eNOS−/− mice14. Inhibitors or solvents were added 30 min before the vessel was contracted with an agonist, and was kept in the solution throughout the experiment. The response elicited by an agonist under baseline conditions was expressed relative to the contraction evoked by 60 mM K+, while that during the contraction evoked by PE (2 μM) was expressed as a change of force relative to the value before the application of the agent.
Assay of COX-derived metabolites
Measurement of the PGI2 metabolite 6-keto-PGF1α, the TxA2 metabolite TxB2, or PGE2 was performed by EIA28,36. Briefly, after being rinsed of blood components, whole sections of aortas were incubated with PSS at 37 °C for 30 min, followed by exposure to PSS (300 μl) and ACh (10 μM) in 300 μl PSS (37 °C) for 15 min each. Thereafter, vessels were taken out, and 1, 10, or 100 μl of reaction solutions was used for 6-keto-PGF1α, PGE2, or TxB2 measurements, respectively (2 replicates for each single measurement), using protocols according to instructions of the manufacturer. The amount of 6-keto-PGF1α, TxB2, or PGE2 was expressed in ng per mg of wet tissue.
Data analysis
Values were expressed as means ± SEM from n numbers or pools of vessels from different animals. The normality of data sets with n of 5 or more was confirmed using the Kolmogorov-Smirnov test. Thereafter, statistical analyses were performed with a Student’s t-test (unpaired) or ANOVA (1-way or 2-way), followed by Bonferroni’s or Dunnett’s post-hoc test. For some data sets with undeterminable normality (n = 3), the Mann-Whitney U test was used. P < 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Li, Z. et al. Role of E-type prostaglandin receptor EP3 in the vasoconstrictor activity evoked by prostacyclin in thromboxane-prostanoid receptor deficient mice. Sci. Rep. 7, 42167; doi: 10.1038/srep42167 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bunting, S., Gryglewski, R., Moncada, S. & Vane, J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins 12, 897–913 (1976).
Davidge, S. T. Prostaglandin H synthase and vascular function. Circ Res 89, 650–660 (2001).
Needleman, P. et al. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature 261, 558–560 (1976).
Smith, W. L., DeWitt, D. L. & Garavito, R. M. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69, 145–182 (2000).
Yu, Y. et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med 4, 132ra154 (2012).
Mitchell, J. A., Ali, F., Bailey, L., Moreno, L. & Harrington, L. S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol 93, 141–147 (2008).
Dusting, G. J., Moncada, S. & Vane, J. R. Prostacyclin (PGI2) is a weak contractor of coronary arteries of the pig. European journal of pharmacology 45, 301–304 (1977).
Gluais, P., Lonchampt, M., Morrow, J. D., Vanhoutte, P. M. & Feletou, M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol 146, 834–845 (2005).
Levy, J. V. Contractile responses to prostacyclin (PGI2) of isolated human saphenous and rat venous tissue. Prostaglandins 16, 93–97 (1978).
Levy, J. V. Prostacyclin-induced contraction of isolated aortic strips from normal and spontaneously hypertensive rats (SHR). Prostaglandins 19, 517–525 (1980).
Rapoport, R. M. & Williams, S. P. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 28, 64–75 (1996).
Zhao, Y. J., Wang, J., Tod, M. L., Rubin, L. J. & Yuan, X. J. Pulmonary vasoconstrictor effects of prostacyclin in rats: potential role of thromboxane receptors. J Appl Physiol (1985) 81, 2595–2603 (1996).
Okon, E. B., Golbabaie, A. & van Breemen, C. In the presence of L-NAME SERCA blockade induces endothelium-dependent contraction of mouse aorta through activation of smooth muscle prostaglandin H2/thromboxane A2 receptors. Br J Pharmacol 137, 545–553 (2002).
Zhou, Y. et al. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol 289, H1027–1032 (2005).
Tang, E. H. et al. Endothelium-dependent contractions occur in the aorta of wild-type and COX2 −/− knockout but not COX1 −/− knockout mice. J Cardiovasc Pharmacol 46, 761–765 (2005).
Katusic, Z. S. & Shepherd, J. T. Endothelium-derived vasoactive factors: II. Endothelium-dependent contraction. Hypertension 18, III86–92 (1991).
Ihara, E. et al. The mechanism of bradykinin-induced endothelium-dependent contraction and relaxation in the porcine interlobar renal artery. Br J Pharmacol 129, 943–952 (2000).
Ihara, E., Hirano, K., Nishimura, J., Nawata, H. & Kanaide, H. Thapsigargin-induced endothelium-dependent triphasic regulation of vascular tone in the porcine renal artery. Br J Pharmacol 128, 689–699 (1999).
Xavier, F. E. et al. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A2 and prostacyclin. Br J Pharmacol 154, 1225–1235 (2008).
Vanhoutte, P. M. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension 57, 526–531 (2011).
Williams, S. P., Dorn, G.W.n. & Rapoport, R. M. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. Am J Physiol 267, H796–803 (1994).
Liu, D. et al. A vasoconstrictor response to COX-1-mediated prostacyclin synthesis in young rat renal arteries that increases in prehypertensive conditions. Am J Physiol Heart Circ Physiol 309, H804–811 (2015).
Baxter, G. S. et al. Characterization of the prostanoid receptors mediating constriction and relaxation of human isolated uterine artery. Br J Pharmacol 116, 1692–1696 (1995).
Farb, M. G. et al. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity (Silver Spring) 22, 349–355 (2014).
Feletou, M., Verbeuren, T. J. & Vanhoutte, P. M. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol 156, 563–574 (2009).
Liu, B. et al. Vasomotor Reaction to Cyclooxygenase-1-Mediated Prostacyclin Synthesis in Carotid Arteries from Two-Kidney-One-Clip Hypertensive Mice. PLoS One 10, e0136738 (2015).
Liu, B. et al. Concomitant activation of functionally opposing prostacyclin and thromboxane prostanoid receptors by cyclo-oxygenase-1-mediated prostacyclin synthesis in mouse arteries. Exp Physiol 97, 895–904 (2012).
Liu, B. et al. Involvement of cyclo-oxygenase-1-mediated prostacyclin synthesis in the vasoconstrictor activity evoked by ACh in mouse arteries. Exp Physiol 97, 277–289 (2012).
Liu, B. et al. Role of Cyclooxygenase-1-Mediated Prostacyclin Synthesis in Endothelium-Dependent Vasoconstrictor Activity of Porcine Interlobular Renal Arteries. Am J Physiol Renal Physiol 302, F1133–1140 (2012).
Liu, B. et al. A vasoconstrictor role for cyclooxygenase-1-mediated prostacyclin synthesis in mouse renal arteries. Am J Physiol Renal Physiol 305, F1315–1322 (2013).
Liu, B. & Zhou, Y. Emerging challenges to the existing paradigm of cyclo-oxygenase pathways in regulating vascular function. Exp Physiol 99, 1–2 (2014).
Eskildsen, M. P. et al. Prostaglandin I2 and prostaglandin E2 modulate human intrarenal artery contractility through prostaglandin E2-EP4, prostacyclin-IP, and thromboxane A2-TP receptors. Hypertension 64, 551–556 (2014).
Tian, X. Y. et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin f(2alpha) impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal 16, 363–373 (2012).
Wong, S. L. et al. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res 104, 228–235 (2009).
Zhou, Y. et al. Cyclo-oxygenase-1 or -2-mediated metabolism of arachidonic acid in endothelium-dependent contraction of mouse arteries. Exp Physiol 98, 1225–1234 (2013).
Li, S. et al. Role of cyclooxygenase-1 and -2 in endothelium-dependent contraction of atherosclerotic mouse abdominal aortas. Clin Exp Pharmacol Physiol 43, 67–74 (2016).
Thomas, D. W. et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest 102, 1994–2001 (1998).
Francois, H., Athirakul, K., Mao, L., Rockman, H. & Coffman, T. M. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 43, 364–369 (2004).
Guan, Y. et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest 117, 2496–2505 (2007).
Hao, C. M. & Breyer, M. D. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70, 357–377 (2008).
Gauthier, K. M. et al. Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations of mouse arteries. Am J Physiol Heart Circ Physiol 300, H725–735 (2011).
Chen, L. et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arteriosclerosis, thrombosis, and vascular biology 32, 3024–3032 (2012).
Morrison, K., Ernst, R., Hess, P., Studer, R. & Clozel, M. Selexipag: a selective prostacyclin receptor agonist that does not affect rat gastric function. J Pharmacol Exp Ther 335, 249–255 (2010).
Luo, W., Liu, B. & Zhou, Y. The endothelial cyclooxygenase pathway: Insights from mouse arteries. European journal of pharmacology 780, 148–158 (2016).
Benyahia, C. et al. A comparative study of PGI2 mimetics used clinically on the vasorelaxation of human pulmonary arteries and veins, role of the DP-receptor. Prostaglandins Other Lipid Mediat 107, 48–55 (2013).
Vachiery, J. L. Prostacyclins in pulmonary arterial hypertension: the need for earlier therapy. Adv Ther 28, 251–269 (2011).
Virdis, A. et al. Endothelial dysfunction in small arteries of essential hypertensive patients: role of cyclooxygenase-2 in oxidative stress generation. Hypertension 62, 337–344 (2013).
Xavier, F. E., Blanco-Rivero, J., Ferrer, M. & Balfagon, G. Endothelium modulates vasoconstrictor response to prostaglandin I2 in rat mesenteric resistance arteries: interaction between EP1 and TP receptors. Br J Pharmacol 158, 1787–1795 (2009).
Tang, E. H. & Vanhoutte, P. M. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics 32, 409–418 (2008).
Bragt, P. C. & Bonta, I. L. Indomethacin inhibits the in vivo formation of the lipoxygenase product HETE (12-hydroxy-5,8,10,14-eicosatetraenoic acid) during granulomatous inflammation in the rat. The Journal of pharmacy and pharmacology 32, 143–144 (1980).
Packham, M. A. & Mustard, J. F. Pharmacology of platelet-affecting drugs. Circulation 62, V26–41 (1980).
Bagi, Z. et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arteriosclerosis, thrombosis, and vascular biology 25, 1610–1616 (2005).
Gluais, P., Vanhoutte, P. M. & Feletou, M. Mechanisms underlying ATP-induced endothelium-dependent contractions in the SHR aorta. European journal of pharmacology 556, 107–114 (2007).
Botella, A., Delvaux, M., Fioramonti, J., Frexinos, J. & Bueno, L. Receptor subtypes involved in dual effects induced by prostaglandin E2 in circular smooth muscle from dog colon. J Pharmacol Exp Ther 273, 1008–1014 (1995).
Fairbrother, S. E., Smith, J. E., Borman, R. A. & Cox, H. M. Characterization of the EP receptor types that mediate longitudinal smooth muscle contraction of human colon, mouse colon and mouse ileum. Neurogastroenterol Motil 23, 782–e336 (2011).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513, 237–241 (2014).
Acknowledgements
We thank Dr. Stanley Lin for critical reading of the MS. This work was supported by the National Natural Science Foundation of China (81470572 and 81270345 to Y Zhou and 81370384 to BL) and by the Department of Education, Guangdong Government under the Top-tier University Development Scheme for Research and Control of Infectious Diseases (to BL).
Author information
Authors and Affiliations
Contributions
Y. Zhou and B.L. conceived and designed the study; Z.L., Y. Zhang, B.L., W.L. and H.L. performed the experiments. Y. Zhou and B.L. analyzed and interpreted data. Y. Zhou wrote the manuscript. Y. Zhou and B.L. provided financial support for the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, Z., Zhang, Y., Liu, B. et al. Role of E-type prostaglandin receptor EP3 in the vasoconstrictor activity evoked by prostacyclin in thromboxane-prostanoid receptor deficient mice. Sci Rep 7, 42167 (2017). https://doi.org/10.1038/srep42167
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep42167
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.