Abstract
Lysine acetylation is a dynamic and reversible post-translational modification that plays an important role in diverse cellular processes. Botrytis cinerea is the most thoroughly studied necrotrophic species due to its broad host range and huge economic impact. However, to date, little is known about the functions of lysine acetylation in this plant pathogen. In this study, we determined the lysine acetylome of B. cinerea through the combination of affinity enrichment and high-resolution LC-MS/MS analysis. Overall, 1582 lysine acetylation sites in 954 proteins were identified. Bioinformatics analysis shows that the acetylated proteins are involved in diverse biological functions and show multiple cellular localizations. Several particular amino acids preferred near acetylation sites, including KacY, KacH, Kac***R, KacF, FKac and Kac***K, were identified in this organism. Protein interaction network analysis demonstrates that a variety of interactions are modulated by protein acetylation. Interestingly, 6 proteins involved in virulence of B. cinerea, including 3 key components of the high-osmolarity glycerol pathway, were found to be acetylated, suggesting that lysine acetylation plays regulatory roles in pathogenesis. These data provides the first comprehensive view of the acetylome of B. cinerea and serves as a rich resource for functional analysis of lysine acetylation in this plant pathogen.
Similar content being viewed by others
Introduction
Lysine acetylation is one of the most common post-translational modifications (PTMs) to proteins in both eukaryotes and prokaryotes. Lysine acetylation, including histone acetylation and non-nuclear protein acetylation, is a dynamic and reversible process. The acetylation status of histones has been extensively studied in the regulation of gene transcription via the modulation of nucleosomal DNA package1,2,3. Furthermore, the discovery of lysine acetylation in non-histone proteins has greatly expanded our understanding of functions of this modification4. To date, protein acetylation has been found to occur in almost every compartments of a cell, such as the cytoplasm and mitochondria5,6. It plays important roles in many cellular physiological processes, including cell-cycle regulation and apoptosis, cell morphology6, metabolic pathways7,8,9,10, protein interactions10 and enzymatic activity9,11. In addition, acetylation has also been implicated in regulating the beneficial effects of calorie restriction, a low nutrient diet without starvation and aging12,13. Therefore, lysine acetylation is believed to be a major signaling modality because of its ubiquitous occurrence and diverse biological functions14,15.
Due to the important role of lysine acetylation in the regulation of protein functions and cellular processes, acetylomes have been determined in many species. Advances in mass spectrometry (MS)-based proteomics have greatly enabled the post-translational modification to be studied on a proteomic scale13,16,17,18. In recent years, the acetylomes of many eukaryotes5,6,13,18,19,20,21,22,23,24 and prokaryotes7,25,26,27,28,29,30,31 have been identified using proteomic methods. These acetylome studies have generated large datasets of PTM sites, which demonstrate the diverse cellular functions of lysine acetylation in these species. The acetylome identification on a large scale greatly increases the knowledge of lysine acetylated proteins and expands the global view of their functional landscape.
Although lysine acetylation is evolutionarily conserved from prokaryotes to eukaryotes6, little is known about the function of this modification in filamentous fungi. Botrytis cinerea, a necrotrophic plant pathogen, can cause grey mold on more than 200 plant species32. The financial impact of grey mold is enormous because B. cinerea causes both pre- and post-harvest losses33. Therefore, it is of considerable interest to examine the regulatory function of lysine acetylation in this important plant pathogen. In this study, we performed the first large-scale analysis of lysine acetylated proteins in B. cinerea. We identified 1582 lysine acetylation sites in 954 proteins which are involved in a variety of biological functions and are localized to multiple cellular compartments. Several particular amino acids preferred near acetylation sites similar to those found in other species were also identified in this organism. Interestingly, 6 proteins involved in virulence of B. cinerea were identified to be acetylated proteins, including 3 key components of high-osmolarity glycerol (HOG) pathway. This work provides the first extensive dataset on lysine acetylation in B. cinerea.
Results
Proteome-wide analysis of lysine acetylation sites and proteins in B. cinerea
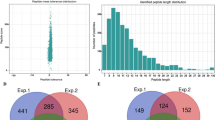
The whole genome of B. cinerea was previously sequenced34, which facilitates systematic analysis of lysine acetylated proteins in this species. To determine the protein acetylome in B. cinerea, a proteomic method was applied. The MS data validation was shown in Fig. 1. The distribution of mass error was near zero and most of them were less than 6 PPM which means the mass accuracy of the MS data fits the requirement (Fig. 1a). In addition, the length of most peptides was distributed between 7 and 19, which agrees with the property of tryptic peptides (Fig. 1b). Therefore, sample preparation reaches the standard. Altogether, 1582 acetylation sites in 954 proteins were identified (Supplementary Table S1), which account for 5.82% (954/16389) of the total proteins in B. cinerea. The acetylated proteins contained different numbers of acetylation sites from 1 to 13 (Fig. 1c). As shown in Fig. 1, 63.5% of the acetylated proteins contain only one acetylation sites and the percentage of proteins with two, three, four or more modification sites are 23%, 6.6% and 6.9%, respectively. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD004031.
Characterization of the lysine acetylome of B. cinerea
To understand the lysine acetylome in B. cinerea, we investigated the GO functional classification of all the identified proteins based on their biological process and molecular function (Fig. 2a,b, Supplementary Table S2, Supplementary Table S3). According to GO annotation information, we calculated the number of identified proteins in each GO term. The classification results for biological process and molecular function showed that enzymes involved in metabolism formed the largest protein group of acetylated proteins, which account for 48 and 44% of the identified proteins, respectively (Fig. 2a,b, Supplementary Table S2, Supplementary Table S3). This is consistent with previous studies that a large proportion of lysine acetylated proteins are categorized as metabolic proteins in other organisms7,25,26,27,28,29. Another large acetylated protein group determined by their molecular function classification is composed of binding proteins, which account for 44% of all the acetyl proteins (Fig. 2b). The second largest group in terms of biological process is proteins associated with cellular processes, which account for 34% of all the identified proteins (Fig. 2a). These results indicate that the acetylated proteins, with diversified molecular functions, are involved in a variety of biological processes in B. cinerea.
The subcellular localization of the acetylated proteins was also analyzed and the results show that they are mainly distributed in the cytosol (35%), nucleus (30%) and mitochondria (19%) (Fig. 2c). The overall trend in the subcellular localization of the acetylated proteins is similar to previous results obtained in Arabidopsis and silkworm22,24.
To better understand the characteristics of the acetylated proteins in B. cinerea, we conducted functional enrichment of GO (biological process, molecular function and cellular component categories), KEGG pathway and protein domain analyses. As determined through the GO and KEGG analyses, proteins involved in ribosome and carbon metabolism were most enriched (Fig. 3, Supplementary Fig. S1, Supplementary Tables S2–S5). In agreement with these observations, the domain enrichment analysis showed that proteins with translation protein SH3-like domain and ribosomal protein L2 domain 2 have a higher tendency to be acetylated (Supplementary Fig. S2, Supplementary Table S6). These results indicate that proteins associated with ribosome are most likely acetylated in B. cinerea. Moreover, the enrichment of pathways in B. cinerea termed microbial metabolism in diverse environments and biosynthesis of secondary metabolites (Fig. 3, Supplementary Table S5) suggests that lysine acetylation may contribute to its pathogenesis and survival under stress conditions.
Based on these findings, we conclude that acetylated proteins have a broad range of biological functions in B. cinerea.
Analysis of acetylated lysine motifs in B. cinerea
In order to understand the properties of acetylation sites, the occupancy frequency of amino acids surrounding the identified modification sites was examined. As shown in Fig. 4, using motif-x program, 1226 peptides (accounted for 77.5% of total peptides identified) were found to include the amino acid sequence from the −10 to the +10 positions surrounding the acetylated lysine. These sequences were matched to a total of ten definitively particular amino acids preferred near acetylation sites, namely, KacY, KacH, Kac***R, KacF, FKac, Kac***K, Kac****R, YKac, LKac and EKac (Fig. 4a), which exhibit different abundances (Fig. 4b) (Kac indicates the acetylated lysine and * indicates a random amino acid residue). Motifs KacY, KacH and KacF were particularly conserved as peptides with these motifs account for approximately 40% of all the identified peptides. Enrichment of residues tyrosine (Y), histidine (H), arginine (R), phenylalanine (F) and lysine (K) were found downstream of the acetylated lysine, whereas tyrosine (Y), glutamic acid (E), leucine (L) and phenylalanine (F) were found upstream of the modification site. For these motifs, the enrichment of aromatic amino acids (Y and F) and a positively charged amino acid (H) was observed in the +1 position. These data suggest that aromatic and positively charged amino acids may be functionally important for acetylation. Moreover, the heat map of the amino acid compositions surrounding the acetylation sites showed that the frequency of K and R in positions −2 to −1 in the motifs is the lowest, whereas the frequency of F, H and Y is the highest (Fig. 4c). Therefore, proteins with such motifs are preferred substrates of lysine acetytransferases in the cell. Among the ten motifs identified in B. cinerea, some were also observed in other eukaryotic and prokaryotic species6,20,21,24,26,28,35, supporting the notion that lysine acetylation is a highly conserved post-translational modification.
Properties of the acetylated peptides.
(a) Acetylation motifs and conservation of acetylation sites. (b) Number of identified modification sites in each acetylated protein. (c) Heat map of the amino acid compositions of the acetylation sites. (d) Probabilities of lysine acetylation in different protein secondary structures (alpha-helix, beta-strand and coil). (e) Predicted surface accessibility of acetylated sites.
To elucidate the relationship between acetylation and protein structure in B. cinerea, the secondary structures of acetylated proteins was investigated in this study. As shown in Fig. 4d, the majority of acetylation sites was located on coils (66.7%) rather than on alpha-helix (26.8%) and beta-strand (6.6%) in B. cinerea (Fig. 4d), which was similar to previous studies24. Although the distribution pattern of acetylated lysine and all lysine is similar (Fig. 4d), there is still tendency of acetylation in B. cinerea based on the P values shown in Fig. 4d. Surface accessibility of acetylated lysine sites was also evaluated. The results showed that approximately 34.8% of acetylated lysine sites were exposed to protein surface, compared with 36.9% of all lysine residues (Fig. 4e). Therefore, lysine acetylation can only slightly affect the surface property of proteins in B. cinerea.
Analysis of protein interaction networks of acetylated proteins in B. cinerea
To further understand the cellular processes regulated by acetylation in B. cinerea, the protein interaction network was established. 954 acetylated proteins were mapped to the protein network database and the global network graph of these interactions was shown in Supplementary Fig. S3 and Supplementary Table S7. Twenty-four highly interconnected clusters of acetylated proteins were retrieved using the Cytoscape software (Fig. 5, Supplementary Table S7). The results showed that acetylated proteins involved in a certain pathway comprise a dense protein interaction network. The subnetwork graphs of acetylated proteins in the top two clusters (ribosome and proteasome) were shown in Fig. 5a,b. Other subnetworks for metabolic pathways, oxidative phosphorylation, microbial metabolism in diverse environments, aminoacyl-tRNA biosynthesis, endoplasmic reticulum and RNA transport were shown in Supplementary Fig. S4 and Supplementary Table S7. These subnetworks also have a relatively high density. These results indicate that the physiological interactions among acetylated protein complexes are likely to contribute to their cooperation in B. cinerea.
Analysis of acetylated proteins involved in virulence of B. cinerea
In this study, six proteins involved in the virulence of B. cinerea were found to be acetylated, including three key components of high-osmolarity glycerol (HOG) pathway: BcSak1, Hpt1 and BOS1 (Table 1). In addition, two chitin synthase, Bcchs2 (Gene Bank accession no. XP_001557191.1) and BcchsV (Gene Bank accession no. XP_001545514) and one polyketide synthase (Gene Bank accession no. XP_001545729.1) which is one of the key enzyme of biosynthesis of the toxic secondary metabolites were also found to be modified by acetyl groups. These results suggest that acetylated proteins may play a role in virulence of B. cinerea.
Discussion
Although lysine acetylation is a widespread and highly conserved post-translational modification in both eukaryotes and prokaryotes with diverse biological functions6, little is known about the function of this modification in filamentous fungi. In this study, we determined the lysine acetylation sites in the proteome of B. cinerea using the combination of affinity enrichment and high-resolution LC-MS/MS analysis. A total of 954 acetylated proteins with 1582 lysine acetylation sites were identified in B. cinerea, which greatly expands the catalog of acetylated proteins in filamentous fungi. Intensive bioinformatics analysis showed that the acetylated proteins are widely distributed and participate in diverse biological processes. The identification of several particular amino acids preferred near acetylation sites suggests substrate preference of lysine acetylation in B. cinerea. Furthermore, protein interaction network analysis demonstrated that a wide range of interactions are modulated by protein acetylation. These data represents the first comprehensive view of the acetylome in B. cinerea.
As a necrotrophic fungus, B. cinerea has the ability to infect many different plant species and tissues under various environmental conditions. Many research efforts have been made in the understanding of its infection strategies36. In this study, six proteins involved in the virulence of B. cinerea were found to be acetylated, including three key components of high-osmolarity glycerol (HOG) pathway: BcSak1, Hpt1 and BOS1 (Table 1). These results indicate that acetylated proteins may play a role in virulence of B. cinerea. In fungi, the HOG pathway is involved in the sensitivity and resistance to fungicides, phenylpyrrole and dicarboximide (e.g., iprodione) and it could be activated by a variety of external stimuli, including fungicides, osmotic stress and hormones37,38,39,40. In B. cinerea, several putative elements of the pathway, including the osmosensor histidine kinase BOS1, the histidine phosphotransfer protein Hpt1, the response regulator BcRrg1, the mitogen-activated protein kinase kinase kinase (MAPKKK) BcOs4, the MAPKK BcOs5 and the MAPK BcSak1 have been identified. To date, all these core elements except for Hpt1, which is essential for B. cinerea, have been characterized. BOS1, BcOs4, BcOs5 and BcSak1 are required for full virulence in B. cinerea41,42,43,44,45. In addition, further studies revealed that the majority of Sak1-regulated genes are unrelated to stress responses, but rather, involved in general metabolic functions46. In support of our observations, p38, an ortholog of the yeast HOG1p MAP kinase (BcSak1 in this study), was found to be acetylated in human. Acetylation of the lysine residue, K53, of p38 protein, regulates the kinase activity of this enzyme47. Although MAPK cascades consist of protein kinases that are sequentially activated by phosphorylation, in this study, the discovery that three key components of HOG pathway were acetylated offers new insights into the understanding of MAP Kinase signal transduction pathways. Additional experiments will be needed to conclusively prove these findings.
Since the penetration structures of B. cinerea need a stable cell wall, several chitin synthase isoenzymes were shown to be important for its pathogenicity48,49. In this study, two chitin synthase, Bcchs2 (Gene Bank accession no. XP_001557191.1) and BcchsV (Gene Bank accession no. XP_001545514), were found to be modified by acetyl groups. These results suggest that acetylation plays an important role in cell wall integrity and virulence of B. cinerea. Moreover, the toxic secondary metabolites play a critical role in host killing. The enzyme responsible for the committed biosynthetic step is often referred to as the “key” enzyme. Forty three genes in B. cinerea were predicted to encode key enzymes: 16 type I iterative Polyketide Synthase, nine Non-Ribosomal Peptide Synthetase, five PKS/NRPS, one DiMethylAllylTryptophan Synthase, one type III PKS (chalcone synthase) and 11 terpene synthases36. Of all the key enzymes, one polyketide synthase (Gene Bank accession no. XP_001545729.1) was identified as acetylated protein, which means lysine acetylation might play a role in secondary metabolism.
In conclusion, we determined the lysine acetylome of B. cinerea for the first time. Our findings showed that acetylated proteins are associated with various biological functions and are distributed in a number of cellular compartments of B. cinerea. Most importantly, several proteins involved in virulence of B. cinerea were found to be modified by acetyl groups. Overall, our results indicate that the regulatory scope of lysine acetylation is broad in B. cinerea.
Methods
Strains and culture
Strain B05.10 of B. cinerea Pers. Fr. [B. fuckeliana (de Bary) Whetzel] is an isolate from Vitis vinifera (Germany) and is widely used as a standard reference strain50. The strain was cultured on PDA (200 g potato, 20 g glucose, 20 g agar and 1 L water) plates. The mycelia were harvested after the strain was grown in potato dextrose broth at 25 °C with shaking at 180 rpm for 2 days.
Protein extraction and digestion
Sample was first ground in liquid nitrogen and sonicated three times on ice using a high intensity ultrasonic processor (Scientz) in lysis buffer (8 M urea, 1% Triton−100, 65 mM DTT and 0.1% Protease Inhibitor Cocktail IV, 3 μm Trichostatin A, 50 mM nicotinamide, 2 mM EDTA). The debris was removed by centrifugation at 20,000 g at 4 °C for 10 min and then the protein was precipitated with 15% cold TCA for 2 h at 4 °C. After centrifugation at 4 °C for 10 min, the supernatant was discarded. After three times wash with cold acetone, the protein was redissolved in buffer (8 M urea, 100 mM NH4CO3, pH 8.0) and the protein concentration was determined with 2-D Quant kit (GE Healthcare) according to the manufacturer’s instructions.
For digestion, the protein solution was reduced with 10 mM DTT for 1 h at 37 °C and then alkylated with 20 mM iodoacetamide for 45 min at room temperature in darkness. For trypsin digestion, the protein sample was diluted by adding 100 mM NH4CO3 to urea concentration less than 2M. Finally, trypsin (Promega) was added at 1:50 trypsin-to-protein mass ratio for the first digestion overnight and 1:100 trypsin-to-protein mass ratio for a second 4 h-digestion.
HPLC fractionation
The sample was fractionated into fractions by high pH reverse-phase HPLC using Agilent 300Extend C18 column (5 μm particles, 4.6 mm ID, 250 mm length). Briefly, peptides were first separated with a gradient of 2% to 60% acetonitrile in 10 mM ammonium bicarbonate pH 10 over 80 min into 80 fractions. Then, the peptides were combined into 8 fractions and dried by vacuum centrifuging.
Affinity enrichment of lysine acetylated peptides
For acetylated peptides enrichment, 10 mg peptides were separated into 8 fraction using HPLC fractionation. Thus, for each fraction, 1.25 mg tryptic peptides dissolved in NETN buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris-HCl, 0.5% NP-40, pH 8.0) were incubated with pre-washed acetyllysine antibody agarose beads (PTM Biolabs) at 4 °C overnight with gentle shaking. The beads were washed four times with NETN buffer and twice with ddH2O. The bound peptides were eluted from the beads with 0.1% trifluoroacetic acid. The eluted fractions were combined and vacuum-dried. The resulting peptides were cleaned with C18 ZipTips (Millipore) according to the manufacturer’s instructions.
Proteomic analysis by LC-MS/MS
Peptides were dissolved in 0.1% formic acid (FA) and directly loaded onto a reversed-phase pre-column (Acclaim PepMap 100, Thermo Scientific). Peptide separation was performed using a reversed-phase analytical column (Acclaim PepMap RSLC, 50 μm × 15 cm, 2 μm, 100 Å, Thermo Scientific). The gradient was comprised of an increase from 7% to 20% solvent B (0.1% FA in 98% acetonitrile) for 20 min, 20% to 35% for 8 min and climbing to 80% in 2 min then holding at 80% for the last 5 min, all at a constant flow rate of 300 nl/min on an EASY-nLC 1000 UPLC system. The composition of the A mobile phase is 0.1% formic acid (FA). The resulting peptides were analyzed by Q ExactiveTM Plus hybrid quadrupole-Orbitrap mass spectrometer (ThermoFisher Scientific).
The peptides were subjected to NSI source followed by tandem mass spectrometry (MS/MS) in Q ExactiveTM Plus (Thermo) coupled online to the UPLC. For MS scans, the m/z scan range was 350 to 1800 and ion charge was set from +2 to +5. Intact peptides were detected in the Orbitrap at a resolution of 70,000. Peptides were selected for MS/MS using NCE setting as 33; ion fragments were detected in the Orbitrap at a resolution of 17,500. A data-dependent procedure that alternated between one MS scan followed by 16 MS/MS scans was applied for the top 16 precursor ions above a threshold ion count of 1.5E4 in the MS survey scan with 10.0 s dynamic exclusion. The electrospray voltage applied was 2.0 kV. Automatic gain control was used to prevent overfilling of the orbitrap; 5E4 ions were accumulated for generation of MS/MS spectra. For MS scans, the m/z scan range was 350 to 1800. Fixed first mass was set as 100 m/z.
Database search
The resulting MS/MS data was processed using MaxQuant with integrated Andromeda search engine (v.1.4.1.2). Tandem mass spectra were searched against UniProt_Botrytis cinerea B05.10 (16,581 sequences) database concatenated with reverse decoy database. Trypsin/P was specified as cleavage enzyme allowing up to 4 missing cleavages, 5 modifications per peptide and 5 charges. Mass error was set to 10 ppm for precursor ions and 0.02 Da for fragment ions. Carbamidomethylation on Cys was specified as fixed modification and oxidation on Met, acetylation on lysine and acetylation on protein N-terminal were specified as variable modifications. False discovery rate thresholds for protein, peptide and modification site were specified at 1%. Minimum peptide length was set at 7. All the other parameters in MaxQuant were set to default values. The site localization probability was set as >0.75. The other parameters were listed below: (1) variable modification: acetyl (protein N-term), Oxidation (Met); (2) fixed modification: carbamidomethyl (Cys); (3) digestion mode: trypisin/P; (4) max missed cleavages: 2; (5) first search PPM: 20; (6) main search PPM: 5; (7) max number of modifications per peptide: 5; (8) min peptide length: 7; (9) min score for modified peptides: 40.
Bioinformatics analyses
Gene Ontology (GO) annotation proteome was derived from the UniProt-GOA database (www. http://www.ebi.ac.uk/GOA/). The identified acetylated proteins were grouped into biological process and molecular function classes based on GO terms using Blast2GO software51. In order to predict subcellular localization, a subcellular localization predication soft Wolfpsort was used in this research52. GO, KEGG pathway and protein domain enrichment were performed using the DAVID bioinformatics resources 6.753. Correction for multiple hypothesis testing was carried out using standard false discovery rate control methods. The GO, KEGG pathway and domain with a corrected p-value < 0.05 were considered significant. Identified proteins domain functional description were annotated by InterProScan (a sequence analysis application) based on protein sequence alignment method using the InterPro domain database54. Soft motif-x was used to analyze the model of sequences constituted with amino acids in specific positions of modifier-21-mers (10 amino acids upstream and downstream of the site) in all protein sequences and a position-specific heat map was generated by plotting the log10 of the ratio using the “heatmap.2” function from the “gplots” R-package55. Secondary structures were predicted using NetSurfP56. The protein-protein interaction networks for the identified acetylated proteins were analyzed by Cytoscape software using interaction data are from the PPI database57.
Additional Information
How to cite this article: Lv, B. et al. Proteome-wide analysis of lysine acetylation in the plant pathogen Botrytis cinerea. Sci. Rep. 6, 29313; doi: 10.1038/srep29313 (2016).
References
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Mutskov, V. et al. Persistent interactions of core histone tails with nucleosomal DNA following acetylation and transcription factor binding. Mol. Cell. Biol. 18, 6293–6304 (1998).
Puig, O., Belles, E., Lopez-Rodas, G., Sendra, R. & Tordera, V. Interaction between N-terminal domain of H4 and DNA is regulated by the acetylation degree. BBA-Gene. Struct. Exp. 1397, 79–90 (1998).
Glozak, M. A., Sengupta, N., Zhang, X. & Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23 (2005).
Kim, S. C. et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 (2006).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Wang, Q. et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327, 1004–1007 (2010).
Guan, K.-L. & Xiong, Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem. Sci. 36, 108–116 (2011).
Nambi, S. et al. Cyclic AMP-dependent protein lysine acylation in mycobacteria regulates fatty acid and propionate metabolism. J. Biol. Chem. 288, 14114–14124 (2013).
Hou, J. et al. Phosphoproteome analysis of rat L6 myotubes using reversed-phase C18 prefractionation and titanium dioxide enrichment. J. Proteome Res. 9, 777–788 (2010).
Starai, V. J. & Escalante-Semerena, J. C. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 340, 1005–1012 (2004).
Imai, S.-i. & Guarente, L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol. Sci. 31, 212–220 (2010).
Henriksen, P. et al. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol. Cell. Proteomics 11, 1510–1522 (2012).
Norris, K. L., Lee, J.-Y. & Yao, T.-P. Acetylation goes global: the emergence of acetylation biology. Sci. Signal. 2, pe76 (2009).
Norvell, A. & McMahon, S. B. Rise of the Rival. Science 327, 964–965 (2010).
Jensen, O. N. Interpreting the protein language using proteomics. Nat. Rev. Mol. Cell Bio. 7, 391–403 (2006).
Choudhary, C. & Mann, M. Decoding signalling networks by mass spectrometry-based proteomics. Nat. Rev. Mol. Cell Bio. 11, 427–439 (2010).
Macek, B., Mann, M. & Olsen, J. V. Global and site-specific quantitative phosphoproteomics: principles and applications. Annu. Rev. Pharmacol. 49, 199–221 (2009).
Zhao, S. et al. Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 (2010).
Lundby, A. et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2, 419–431 (2012).
Weinert, B. T. et al. Proteome-wide mapping of the Drosophila acetylome demonstrates a high degree of conservation of lysine acetylation. Sci. Signal. 4, ra48 (2011).
Finkemeier, I., Laxa, M., Miguet, L., Howden, A. J. & Sweetlove, L. J. Proteins of diverse function and subcellular location are lysine acetylated in Arabidopsis. Plant Physiol. 155, 1779–1790 (2011).
Nallamilli, B. et al. Global analysis of lysine acetylation suggests the involvement of protein acetylation in diverse biological processes in rice (Oryza sativa). PLoS ONE 9, e89283 (2014).
Nie, Z. et al. Comprehensive profiling of lysine acetylation suggests the widespread function is regulated by protein acetylation in the silkworm, Bombyx mori. Proteomics 15, 3253–3266 (2015).
Yu, B. J., Kim, J. A., Moon, J. H., Ryu, S. E. & Pan, J.-G. The diversity of lysine-acetylated proteins in Escherichia coli. J. Microbiol. Biotechn. 18, 1529–1536 (2008).
Zhang, J. et al. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol. Cell. Proteomics 8, 215–225 (2009).
Zhang, K., Zheng, S., Yang, J. S., Chen, Y. & Cheng, Z. Comprehensive profiling of protein lysine acetylation in Escherichia coli. J. Proteome Res. 12, 844–851 (2013).
Pan, J. et al. Systematic analysis of the lysine acetylome in Vibrio parahemolyticus. J. Proteome Res. 13, 3294–3302 (2014).
Okanishi, H., Kim, K., Masui, R. & Kuramitsu, S. Acetylome with structural mapping reveals the significance of lysine acetylation in Thermus thermophilus. J. Proteome Res. 12, 3952–3968 (2013).
Ouidir, T., Cosette, P., Jouenne, T. & Hardouin, J. Proteomic profiling of lysine acetylation in Pseudomonas aeruginosa reveals the diversity of acetylated proteins. Proteomics 15, 2152–2157 (2015).
Liu, F. et al. Acetylome analysis reveals diverse functions of lysine acetylation in Mycobacterium tuberculosis. Mol. Cell. Proteomics 13, 3352–3366 (2014).
Williamson, B., Tudzynski, B., Tudzynski, P. & van Kan, J. A. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580 (2007).
Dean, R. et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430 (2012).
Amselem, J. et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7, e1002230 (2011).
Shao, J. et al. Systematic analysis of human lysine acetylation proteins and accurate prediction of human lysine acetylation through bi-relative adapted binomial score Bayes feature representation. Mol. BioSyst. 8, 2964–2973 (2012).
Hahn, M., Viaud, M. & van Kan, J. The genome of Botrytis cinerea, a ubiquitous broad host range necrotroph in Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens (eds Dean, R. A., Lichens-Park, A. & Kole, C. ) Ch. 2, 19–44 (Springer, 2014).
Xu, J. R. MAP kinases in fungal pathogens. Fungal Genet. Biol. 31, 137–152 (2000).
Jones, C. A., Greer-Phillips, S. E. & Borkovich, K. A. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress and fungicide resistance in Neurospora crassa. Mol. Biol. Cell 18, 2123–2136 (2007).
Eisman, B. et al. The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryot. Cell 5, 347–358 (2006).
Boisnard, S. et al. Insight into the role of HOG pathway components Ssk2p, Pbs2p and Hog1p in the opportunistic yeast Candida lusitaniae. Eukaryot. Cell 7, 2179–2183 (2008).
Segmüller, N., Ellendorf, U., Tudzynski, B. & Tudzynski, P. BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot. Cell 6, 211–221 (2007).
Yang, Q., Yan, L., Gu, Q. & Ma, Z. The mitogen-activated protein kinase kinase kinase BcOs4 is required for vegetative differentiation and pathogenicity in Botrytis cinerea. Appl. Microbiol. Biot. 96, 481–492 (2012).
Yan, L., Yang, Q., Jiang, J., Michailides, T. J. & Ma, Z. Involvement of a putative response regulator Brrg-1 in the regulation of sporulation, sensitivity to fungicides and osmotic stress in Botrytis cinerea. Appl. Microbiol. Biot. 90, 215–226 (2011).
Viaud, M. et al. A class III histidine kinase acts as a novel virulence factor in Botrytis cinerea. Mol. Plant Microbe In. 19, 1042–1050 (2006).
Liu, W., Leroux, P. & Fillinger, S. The HOG1-like MAP kinase Sak1 of Botrytis cinerea is negatively regulated by the upstream histidine kinase Bos1 and is not involved in dicarboximide-and phenylpyrrole-resistance. Fungal Genet. Biol. 45, 1062–1074 (2008).
Heller, J. et al. The mitogen-activated protein kinase BcSak1 of Botrytis cinerea is required for pathogenic development and has broad regulatory functions beyond stress response. Mol. Plant Microbe In. 25, 802–816 (2012).
Pillai, V. B. et al. Acetylation of a conserved lysine residue in the ATP binding pocket of p38 augments its kinase activity during hypertrophy of cardiomyocytes. Mol. Cell. Biol. 31, 2349–2363 (2011).
Morcx, S. et al. Disruption of Bcchs4, Bcchs6 or Bcchs7 chitin synthase genes in Botrytis cinerea and the essential role of class VI chitin synthase (Bcchs6). Fungal Genet. Biol. 52, 1–8 (2013).
Arbelet, D. et al. Disruption of the Bcchs3a chitin synthase gene in Botrytis cinerea is responsible for altered adhesion and overstimulation of host plant immunity. Mol. Plant Microbe In. 23, 1324–1334 (2010).
Quidde, T., Büttner, P. & Tudzynski, P. Evidence for three different specific saponin-detoxifying activities in Botrytis cinerea and cloning and functional analysis of a gene coding for a putative avenacinase. Eur. J. Plant Pathol. 105, 273–283 (1999).
Conesa, A. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676 (2005).
Horton, P. et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 35, W585–W587 (2007).
Jiao, X. et al. DAVID-WS: a stateful web service to facilitate gene/protein list analysis. Bioinformatics 28, 1805–1806 (2012).
Quevillon, E. et al. InterProScan: protein domains identifier. Nucleic Acids Res. 33, W116–W120 (2005).
Chou, M. F. & Schwartz, D. Biological sequence motif discovery using motif‐x. Curr. Protoc. Bioinformatics. 13.15. 11–13.15. 24 (2011).
Petersen, B., Petersen, T. N., Andersen, P., Nielsen, M. & Lundegaard, C. A generic method for assignment of reliability scores applied to solvent accessibility predictions. BMC Struct. Biol. 9, 51 (2009).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
This wok was supported by the National Natural Science Foundation of China (31370779, 31401027, 31501590), the Taishan Scholar Construction Foundation of Shandong Province (6631114314) and the Program for Talents in Qingdao Agricultural University (6631114307, 6631115038).
Author information
Authors and Affiliations
Contributions
W.L. and S.L. designed research; B.L., Q.Y. and D.L. performed research; B.L., Q.Y., D.L., W.L. and L.S. analyzed data; B.L., Q.Y., D.L., W.L. and L.S. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lv, B., Yang, Q., Li, D. et al. Proteome-wide analysis of lysine acetylation in the plant pathogen Botrytis cinerea. Sci Rep 6, 29313 (2016). https://doi.org/10.1038/srep29313
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29313
This article is cited by
-
Computational investigation on the effect of the lysine 2-hydroxyisobutyrylation on argininosuccinate synthetase 1 conformational dynamics in Botrytis cinerea
Journal of Molecular Modeling (2023)
-
Tandem Mass Tag labelling quantitative acetylome analysis of differentially modified proteins during mycoparasitism of Clonostachys chloroleuca 67–1
Scientific Reports (2021)
-
Lysine acetylation as drug target in fungi: an underexplored potential in Aspergillus spp.
Brazilian Journal of Microbiology (2020)
-
Systematic analysis of the lysine succinylome in the model medicinal mushroom Ganoderma lucidum
BMC Genomics (2019)
-
Novel proteome and acetylome of Bemisia tabaci Q in response to Cardinium infection
BMC Genomics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.