Abstract
The Stac3 gene is exclusively expressed in skeletal muscle, and Stac3 knockout is perinatal lethal in mice. Previous data from Stac3-deleted diaphragms indicated that Stac3-deleted skeletal muscle could not contract because of defective excitation-contraction (EC) coupling. In this study, we determined the contractility of Stac3-deleted hindlimb muscle. In response to frequent electrostimulation, Stac3-deleted hindlimb muscle contracted but the maximal tension generated was only 20% of that in control (wild type or heterozygous) muscle (P < 0.05). In response to high [K+], caffeine, and 4-chloro-m-cresol (4-CMC), the maximal tensions generated in Stac3-deleted muscle were 29% (P < 0.05), 58% (P = 0.08), and 55% (P < 0.05) of those in control muscle, respectively. In response to 4-CMC or caffeine, over 90% of myotubes formed from control myoblasts contracted, but only 60% of myotubes formed from Stac3-deleted myoblasts contracted (P = 0.05). However, in response to 4-CMC or caffeine, similar increases in intracellular calcium concentration were observed in Stac3-deleted and control myotubes. Gene expression and histological analyses revealed that Stac3-deleted hindlimb muscle contained more slow type-like fibers than control muscle. These data together confirm a critical role of STAC3 in EC coupling but also suggest that STAC3 may have additional functions in skeletal muscle, at least in the hindlimb muscle.
Similar content being viewed by others
Introduction
Skeletal muscle contraction is essential for movement, posture, heat generation, and respiration in mammals. Skeletal muscle contraction begins with the transmission of action potentials from the motor nerve causing the release of acetylcholine (ACh) from the pre-synaptic terminals. The released ACh binds to its receptor (AChR) on the sarcolemma1,2. Sodium influx through the AChR depolarizes the sarcolemma and subsequently causes the generation of action potentials. Action potentials spread along the transverse tubules (T-tubules) into the muscle fibers3,4. It is believed that in response to action potentials transmitted from the T-tubules, the dihydropyridine receptors (DHPR) on the T-tubules change their conformation and cause the calcium channel ryanodine receptors (RyR) on the sarcoplasmic reticulum (SR) to open and release calcium into the sarcoplasm5,6,7,8. The increased sarcoplasmic calcium triggers the myofibrils to contract, completing the process termed excitation-contraction (EC) coupling9.
The Stac3 gene, which encodes a protein containing a Src homology 3 (SH3) domain and a cysteine rich (C1) domain, is a member of the Stac gene family10. The Stac3 gene is predominantly expressed in skeletal muscle11,12. Transgenic animal studies demonstrate that STAC3 is essential for skeletal muscle contraction and postnatal life in mice11,12 and that STAC3 plays an important role in skeletal muscle contraction in zebrafish13. A recent study demonstrates that STAC3 can target the DHPR α1 subunit to cell membrane14. In humans, two congenital myopathies, Native American myopathy13 and King-Denborough syndrome15, have been associated with mutations in the Stac3 gene. In vitro studies using primary mouse myoblasts, C2C12 cells, and bovine satellite cells indicated that STAC3 might also play a role in myoblast differentiation16,17,18.
The diaphragm muscle from Stac3 knockout mice did not contract in response to electric stimulation but generated normal tension when stimulated by the RyR agonist 4-CMC11. Based on this data, it was concluded that Stac3 knockout impaired skeletal muscle contraction by disrupting EC coupling11. The diaphragm muscle, however, is different from limb muscles. For example, the diaphragm differs from the hindlimb muscles in the type of RyR expressed19. Two RyR isoforms, RyR1 and RyR3, are expressed in skeletal muscle, and they have different sensitivities to the RyR agonists, 4-chloro-m-cresol (4-CMC) and caffeine20,21,22,23. While RyR1 is the predominant RyR expressed in the hindlimb muscles, both RyR1 and RyR3 are expressed at high levels in the diaphragm24. The diaphragm and hindlimb muscles also differ in the type of myosin heavy chain (MHC) protein expressed. The gene encoding the major fast MHC protein Myh4 is not expressed in the diaphragm, but it is abundantly expressed in the hindlimb muscles25. These and other differences between the diaphragm and hindlimb muscles prompted us to examine the effect of Stac3 knockout on the contractility of hindlimb skeletal muscles in mice. Our data indicate that while EC coupling is impaired, it is not the sole cause for the markedly reduced contractility in Stac3-deleted hindlimb muscles.
Results
Response of Stac3-deleted hindlimb muscles to electrostimulation
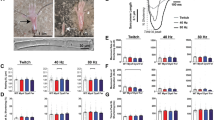
When stimulated by single electric pulses, E18.5 Stac3−/− hindlimb muscles did not contract whereas E18.5 Stac3+/− hindlimb muscles generated twitches (Fig. 1A). When stimulated by repeated electric pulses, Stac3+/− muscles demonstrated typical tetanic contraction (Fig. 1A). When stimulated by repeated electric pulses, Stac3−/− muscles contracted, but the maximal tension generated was only 20% of that in Stac3+/− muscles (Fig. 1A,B). One second after the stimulation was stopped, Stac3+/− muscles completely relaxed but Stac3−/− muscles remained in contraction (Fig. 1A).
Hindlimb muscle preparations were stimulated by a single electric pulse or repeated pulses of 500 μsec and 20 v, and tension was recorded with a force transducer. (A) Representative recordings of twitch and tetanic tension. Upper trace indicates tension changes and lower trace indicates electric stimulation. (B) Tension-frequency relationships. Maximum tension was normalized to muscle mass. Data are presented as mean ± SEM (n = 9 mice). **P < 0.01, between the genotypes at the same frequency. Tensions labeled with different letters are different (P < 0.05) within the same genotype.
Response of Stac3-deleted hindlimb muscles to RyR agonists
To determine the possibility that Stac3-deleted hindlimb muscles had defective EC coupling, we tested the contractile responses of the muscles to high [K+]-induced depolarization and 4-CMC and caffeine activation of RyR. Consistent with the responses to electrostimulation (Fig. 1), 80 mM [K+]-induced maximal tension in Stac3−/− hindlimb muscles was only 29% of that in Stac3+/− muscles (Fig. 2A,B). In response to 5 mM 4-CMC and 25 mM caffeine (Fig. 2A,B), the maximal tensions generated in Stac3−/− muscles were 58% (P = 0.08) and 55% (P < 0.05) of those generated in Stac3+/− muscles, respectively (Fig. 2A,B). These percentages were, however, much greater than those associated with electrostimulation (20%, Fig. 1) and high [K+] (29%, Fig. 2). Based on these percentages, nearly 60% of the lost contractility in Stac3−/− hindlimb muscles was due to defective EC coupling (from sarcolemma to SR) while the remaining 40% was due to post EC coupling defects. The maximal tension induced by 1 mM 4-CMC in Stac3−/− muscles was not different from that in Stac3+/− muscles (Fig. 2). The tension induced by 1 mM 4-CMC in Stac3+/− muscles was only 70% (P < 0.05) of that induced by 5 mM 4-CMC or 50% (P < 0.05) of that induced by 25 mM caffeine in the same Stac3+/− muscles (Fig. 2B).
(A) Representative recordings of muscle tension. Arrows indicate when potassium (80 mM), 4-CMC (1 or 5 mM), or caffeine (25 mM) was applied to the muscle. (B) Maximum tension normalized to muscle mass. Data = mean ± SEM (n = 4 mice per genotype for potassium and 4-CMC; n = 5 for caffeine). *P < 0.05.
Spontaneous and RyR agonists-induced contraction in Stac3-deleted myotubes
We compared the contractility of myotubes formed from E18.5 Stac3−/− and Stac3+/− myoblasts (Fig. 3A). Over 30% of myotubes formed from Stac3+/− myoblasts displayed spontaneous contraction, whereas none of those formed from Stac3−/− myoblasts was observed to contract (Fig. 3B). In response to 5 mM 4-CMC or 25 mM caffeine, 90% of Stac3+/− myotubes contracted, but only 60% of Stac3−/− myotubes contracted (P = 0.05) (Fig. 3B,D). These data further indicated that defective EC coupling was a major reason but not the only reason that Stac3-deleted myotubes could not contract. Interestingly, among the Stac3−/− myotubes that contracted in response to 4-CMC or caffeine treatment, approximately 50% of them detached from the plates following the contraction, whereas only 10% of Stac3+/− myotubes detached following 4-CMC or caffeine-stimulated contraction (Fig. 3E).
Myoblasts were isolated from hindlimbs of E18.5 Stac3+/− or Stac3−/− fetuses and induced to differentiate into myotubes. Spontaneous contraction was determined by examining 1-min videos of unstimulated myotubes. 4-CMC or caffeine-stimulated contraction in myotubes was determined by examining videos of myotubes 5 seconds before and 1 minute after the addition of 5 mM 4-CMC or 25 mM caffeine to the culture medium. (A) Representative photographs of Stac3+/− and Stac3−/− myotubes. Scale bars = 200 μm. (B) Representative images of myotubes immediately before and after 4-CMC or caffeine treatment. Arrows point to contracting myotubes. (C) Percentage of spontaneously contracting myotubes. (D) Percentages of 4-CMC and caffeine-induced contracting myotubes. (E) Percentages of detached and attached myotubes that contracted in response to 4-CMC or caffeine. Data = mean ± SEM (n = 4 mice per genotype). **P < 0.01.
RyR agonists-induced increases in intracellular calcium in Stac3-deleted myotubes
Reduced contractility in Stac3-deleted skeletal muscle could be due to reduced function of RyR or reduced calcium storage in the SR. We determined these possibilities by comparing 4-CMC- and caffeine-induced increases in intracellular calcium in Stac3+/− and Stac3−/− myotubes (Fig. 4A). As can be seen from Fig. 4B, neither the concentration of intracellular calcium before nor that after stimulation by 5 mM 4-CMC or 25 mM caffeine was different between Stac3+/− and Stac3−/− myotubes (Fig. 4B). These data indicated that Stac3 knockout did not affect the function of RyR or the amount of releasable calcium in the SR.
Myotubes were loaded with the fluorescent calcium indicator Fura-2. Fluorescence emission activated by light at 340 nm and 380 nm was recorded by a microscope. (A) Representative images of Stac3+/− and Stac3−/− myotubes loaded with Fura-2 at 3 seconds before and 1 second after 5 mM 4-CMC or 25 mM caffeine treatment. (B) Resting and 4-CMC- and caffeine-induced peak intracellular calcium concentrations. Data = mean ± SEM (n ≥ 7 myotubes from 4 mice per genotype).
Fiber composition in Stac3-deleted hindlimb muscles
Based on myosin-ATPase and NADH-TR staining, E18.5 Stac3−/− hindlimb muscles contained more slow fiber-like myotubes than Stac3+/+ hindlimb muscles (Fig. 5A,B). Myosin-ATPase staining also revealed that myonuclei, which did not contain myosin and hence stained pale, were localized peripherally in Stac3+/+ myotubes but centrally in Stac3−/− myotubes (Fig. 5A). RT-qPCR analyses revealed that slow fiber markers, such as troponin T type 1 (Tnnt1), myosin heavy chain 7 (Myh7), myocyte specific enhancer factor 2C (Mef2c), peroxisome proliferator-activated receptor gamma coactivator 1 alpha (Ppargc1a), and myogenin (Myog) were expressed at greater levels in Stac3−/− than Stac3+/+ muscles, whereas fast fiber markers such as Tnnt3, Myh3, Myh4, Myh8, and alpha actinin 3 (Actn3) were expressed at lower levels in Stac3−/− than Stac3+/+ muscles (Table 1). Overall, these histological and gene expression data indicated that hindlimb muscles from Stac3−/− fetuses were more like slow muscles and less like fast muscles than those from Stac3+/+ fetuses.
Discussion
Our previous study showed that the mouse Stac3 gene is specifically expressed in skeletal muscle and that Stac3 knockout mice died at birth12. Our present study showed that hindlimb muscles from Stac3-deleted mouse fetuses barely contracted in response to electrostimulation or high [K+]-induced membrane depolarization but that they contracted in response to the RyR agonists 4-CMC and caffeine. These results demonstrate that Stac3-deleted hindlimb muscles were defective in EC coupling, a finding consistent with that from Stac3-deleted diaphragm11. However, our study also showed that 4-CMC or caffeine-induced maximal tension in Stac3-deleted hindlimb muscles was only 60% of that in normal hindlimb muscles. This result indicates that although defective EC coupling was the major cause, it was not the only cause for the lost contractibility in Stac3-deleted hindlimb muscles. In other words, both EC coupling and post EC coupling defects, which could be troponin Ca2+ binding defects, less ATP, or defective myofibril protein assembly or expression, contributed to the reduced contractility in Stac3-deleted hindlimb muscles.
Discrepant from our study, a post EC coupling defect was not suggested by the response of the Stac3-deleted diaphragm to 4-CMC11. This discrepancy could be due to different concentrations of 4-CMC used between the studies. In the study by Nelson et al., 1 mM 4-CMC elicited similar contraction in Stac3-deleted and normal diaphragms11. In the present study, we found that while tension induced by 1 mM 4-CMC in Stac3-deleted hindlimb muscles was not different from that induced by 5 mM 4-CMC or 25 mM caffeine, tension induced by 1 mM 4-CMC in normal muscles was only 70% of that induced by 5 mM 4-CMC or 50% of that induced by 25 mM caffeine in the same muscles. Our result suggests that 1 mM 4-CMC is insufficient to reveal differences in maximal tension between Stac3-deleted and normal muscles and hence insufficient to reveal post EC coupling defects in Stac3-deleted muscles. It remains to be determined why it takes less 4-CMC to induce maximal tension in Stac3-deleted muscles than in normal muscles. We speculate that either Stac3-deleted muscles are more permeable to 4-CMC than normal muscles or that ryanodine receptors are more sensitive to 4-CMC in Stac3-deleted muscles than in normal muscles. A membrane difference between Stac3-deleted and normal muscles is suggested by the observation that Stac3-deleted myotubes were more inclined to detach from the plates than control myotubes upon contraction. The possibility that Stac3 deletion increased the sensitivity of ryanodine receptors to 4-CMC is, however, not supported by the data that 4-CMC-induced increase in intracellular calcium was not different between Stac3-deleted and control myofibers. Two RyR isoforms, RyR1 and RyR3, are expressed in skeletal muscle8. RyR3 and RyR1 differ in sensitivity to 4-CMC and caffeine22,23. The hindlimb muscles express RyR1, but the diaphragm expresses RyR3 in addition to RyR18,24. Mouse hindlimb muscles contain predominantly fast fibers instead of the mixture of slow fiber and fast fiber in the diaphragm19. These differences in RyR isoform and fiber type could also contribute to the different responses of hindlimb muscles and diaphragm to 4-CMC and caffeine.
Theoretically, reduced RyR expression or function, reduced SR storage of calcium, reduced calcium binding capacity of troponin C, reduced ATP availability, and reduced function of contractile apparatus could each reduce the contractility of skeletal muscle. Our study showed that in response to the RyR agonists 4-CMC and caffeine, similar amounts of calcium were released from the SR in Stac3-deleted and control myotubes. This result, consistent with the finding from Stac3-deleted diaphragm11, does not support the possibility that Stac3 deletion reduces muscle contractility by reducing the function of RyR or the amount of releasable calcium stored in the SR. In this study, we found that hindlimb muscles from Stac3-deleted mouse fetuses were more like slow muscles than control hindlimb muscles. This result supports the possibility that Stac3 deletion stimulated slow fiber differentiation or inhibited fast fiber differentiation and thereby reduced muscle contractibility because fast muscles contract with greater force than slow muscles19. Reduced muscle activity would stimulate fiber differentiation toward fast muscle26,27,28. Thus, it is unlikely that the effect of Stac3 deletion on fiber type differentiation was secondary to that on muscle contractility. Skeletal muscle of neonatal Stac3-deleted mice contained an abnormally high percentage of centrally localized nuclei and disorganized myofibrils12. These abnormalities may be or may be not related to lack of contraction. The SH3 domain is a versatile protein-protein interaction domain29,30. It is plausible to speculate that while STAC3 mediates EC coupling through interaction with DHPR13,14, it mediates fiber differentiation, myofibril assembly, and nuclear location through other unknown protein partners.
Methods
Animals and genotyping
The generation of heterozygous Stac3 mutant mice has been described before12. These mice were mated to generate homozygous Stac3 mutant mice. Mice were genotyped as previously described12. Homozygous Stac3 mutant (Stac3−/−), heterozygous (Stac3+/−) and wild-type (Stac3+/+) mouse fetuses at embryonic day 18.5 (E18.5) or E17.5 were used in this study. All protocols involving mice were approved by the Virginia Tech Institutional Animal Care and Use Committee and were carried out in accordance with the approved guidelines.
Muscle contractility tests
Pregnant mice were euthanized by CO2 inhalation followed by cervical dislocation. The fetuses were quickly dissected out of the uterus and euthanized by decapitation on ice. The right hindlimb was skinned and separated from the body at the hip and knee joints in physiological salt solution (PSS: 150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES pH 7.4, and 5.6 mM glucose). The hindlimb preparation was then incubated in a chamber of PSS between a clamp and an arm of Dual-Mode Level Servomotor System (300B, Aurora Scientific Inc., Aurora, Ontario, Canada). The PSS was maintained at 30 °C and was constantly bubbled with 95% O2 and 5% CO2 as previously described31. The servomotor arm and stepper motor were controlled by the Dynamic Muscle Control software (DMC Version 4.1.6, Aurora Scientific) to obtain the tension output data.
A pair of Stac3−/− and Stac3+/− hindlimb muscle preparations were tested at the same time. The treatments were electric stimulation (20 v, 500 μsec; single or frequent), 80 mM KCl, 1 mM and 5 mM 4-CMC, and 25 mM caffeine. The concentrations of KCl, 4-CMC, and caffeine used in this study were based on previous studies11,32. There was a 1 min interval between two consecutive electric stimulations. There was a 5 min PSS wash out interval between two chemical treatments. Resting tension was set at 0.5 g. At the end of each test, muscles were gently blotted dry with Kimwipes and weighed to the nearest 0.1 mg using an A-200D electronic analytical balance (Denver Instruments, Denver, Colorado). Twitch and tetanic tensions were normalized to muscle mass.
Myoblast isolation and culture
This procedure was performed as previously described33,34. Hindlimb muscles from E18.5 mouse fetuses were minced with razors and digested in Ham’s F-10 medium (2 ml/g tissue) supplemented with 1.5 U/ml collagenase B (Roche Diagnostics Corp., Indianapolis, Indiana), 2.4 U/ml dispase (Roche), and 2.5 mM CaCl2 at 37 °C for 2 × 10 min. The tissue slurry was gently pipetted to release cells between the two digestions. At the end of the second 10-min digestion, the tissue slurry was mixed with 10 volumes of Ham’s F-10 medium supplemented with 0.1% FBS (Atlanta biologicals, Norcross, Georgia) and passed through a 40 μm nylon filter. The filtrate was centrifuged at 350 × g for 5 min. The pelleted cells were resuspended in myoblast culture medium consisting of Ham’s F-10 medium, 20% FBS, 5 ng/ml bFGF (Thermo Fisher Scientific Inc., Waltham, Massachusetts), and antibiotic-antimycotic (ABAM) (Thermo Fisher Scientific), and plated on a non-coated dish for 20 min to allow the fibroblasts to attach. The supernatant in the dish, which contained unattached primary myoblasts, was then transferred to a collagen type I (Sigma-Aldrich, St. Louis, Missouri) coated dish and cultured in a 37 °C incubator with 5% CO2 for 24 h. Subsequently, the culture medium was transferred to a 15 ml centrifuge tube while 1 ml of PBS was added to the same dish. The dish was hit firmly against a table top to dislodge the cells. The dish was treated this way for 5–10 min to allow all myoblasts to disassociate from the dish. All the liquid from the dish was collected to the original 15 ml tube. The collection was centrifuged at 350 × g for 5 min. The cell pellet was resuspended with myoblast culture medium and plated to a new collagen-coated dish. This preplating procedure was repeated 3 times to enrich myoblasts.
Myotube contractility analysis
Myoblasts prepared above were plated at 60% confluence on collagen coated 24-well plates and induced to differentiate in medium composed of DMEM, 2% horse serum, and ABAM. Videos of myotubes were taken using a CKX41 inverted microscope connected to a QColor3 OLYMPUS digital camera (Olympus Corp., Shinjuku, Tokyo, Japan). To observe spontaneous contraction in myotubes, 1-minute videos were taken of Stac3+/− and Stac3−/− myotubes in differentiation medium. To determine the effect of 4-CMC and caffeine on myotube contraction, 5 mM 4-CMC or 25 mM caffeine was added to the medium immediately before 1-minute videos were taken. Contracting or non-contracting myotubes were counted by analyzing the videos. Myotubes that detached from the plates at one end or both ends as a result of contraction were considered as detached myotubes, and those s that remained attached to the plate at both ends following contraction were considered as attached myotubes.
Calcium imaging
This procedure was performed as previously described35,36. Briefly, myotubes formed above were loaded with 5 μM fura-2 AM (Thermo Fisher Scientific), a fluorescent calcium indicator, for 40 min in the dark at 37 °C. Myotubes were then rinsed with pre-warmed DMEM twice, each for 10 min. Myotubes were incubated in calcium-free ringer buffer (100 mM NaCl, 6 mM KCl, 2 mM NaHCO3, pH 7.4) for 30 min at room temperature to allow complete de-esterification of intracellular AM esters. Fluorescence was measured using an Eclipse Ti fluorescent microscope system with the pre-set fura-2 channel (Nikon Corp., Chiyoda, Tokyo, Japan). At least 3 myotubes were imaged at the same time. To induce the release of calcium from the SR, myotubes were treated with 5 mM 4-CMC or 25 mM caffeine, and the fluorescence was recorded from 1 min before to 5 min after the treatment was added. Intracellular calcium concentration was calculated as the ratio of fura-2 fluorescence excited by ultraviolet light at 340 nm (calcium bound) to that excited at 380 nm (calcium unbound). The 340 nm to 380 nm ratio before the treatment was used as the resting ratio.
Myosin-ATPase and nicotinamide adenine dinucleotide-tetrazolium reductase (NADH-TR) staining
Left hindlimbs from E18.5 mouse fetuses were immersed in optimal cutting temperature compound (OCT) on a cork sheet and were immediately frozen in liquid nitrogen pre-cooled isopentane. Bones were kept to help locate limb muscles. The muscle samples were left in a Leica CM 1800 cryostat at −20 °C for 20 min for thermal adaptation. Cross-sections of 8 μm thick were cut and mounted on Fisher Superfrost Plus slides (Thermo Fisher Scientific). Myosin-ATPase staining was performed as described37. Briefly, muscle sections were pre-incubated for 15 min in sodium barbital buffer containing 20 mM sodium barbital and 36 mM CaCl2 at pH 10.2 and then incubated for 45 min in sodium barbital buffer containing 3.5 mM ATP, 20 mM sodium barbital, and 18 mM CaCl2 at pH 9.4. Muscle sections were rinsed in 1% CaCl2 for 3 × 1 min, and then immersed in 2% CoCl2 for 2 min. The sections were then stained with 1% (NH4)2S for 20 sec followed by 5 rinses with ddH2O. Stained sections were dehydrated in 50%, 70%, 80%, 90% and 100% ethanol, cleared in xylene, and then mounted in 50% xylene and 50% Canada balsam mixture (Thermo Fisher Scientific).
The NADH-TR staining was performed as previously described37. Briefly, muscle sections were incubated at 37 °C for 10 min and then with 1 mg/ml nitroblue tetrazolium (Sigma-Aldrich) and 0.4 mg/ml β-NADH (Sigma-Aldrich) in 50 mM Tris-HCl buffer, pH 7.3, at 37 °C for 30 min. After 5 × 5 min washes in a graded acetone series (30%, 60%, 90%, 60% and 30%), muscle sections were washed with ddH2O and mounted in prolong gold mounting medium (Life Technologies Corp., Carlsbad, California) and cover-slipped for imaging with an Eclipse TS100 microscope (Nikon Corp.).
Total RNA isolation and RT-qPCR
Hindlimb muscles from E17.5 mouse fetuses were homogenized in TRI reagent (Molecular Research Center, Inc., Cincinnati, Ohio) using a TissueLyser II (Qiagen, Hilden, Germany). The remaining steps followed the manufacturer’s instruction (Molecular Research Center). Concentration and purity of extracted RNA were determined using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific). For first-strand cDNA synthesis, 0.5 μg of total RNA and 0.5 μg random primer (Promega Corp., Madison, Wisconsin) were adjusted to 5 μl with DEPC-treated water and heated to 70 °C for 5 min to denature the RNA. The RNA-primer mixture was then added with 4 μl of 5× reverse transcription reaction buffer, 4.8 μl of 25 mM MgCl2, 3.7 μl of DEPC-treated water, 1 μl of 10 mM dNTP, 0.5 μl of RNase inhibitor and 1 μl of ImProm-II reverse transcriptase (Promega). The reaction was incubated at 25 °C for 5 min, 42 °C for 60 min, and then at 70 °C for 15 min. Quantitative PCR (qPCR) was performed on 1 μl of cDNA product in a total volume of 10 μl containing 5 μl of SyberGreen PCR Master Mix (Life Technologies Corp.) and 0.2 μM gene-specific forward and reverse primers (Table 2) under 36 cycles of 15 sec at 95 °C and 1 min at 60 °C on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, California). Primers were designed using the Primer3 software (MIT, Cambridge, Massachusetts). The specificity of all primers was verified by 2% agarose gel electrophoresis and DNA sequencing of PCR products. The efficiency of all primers was determined by analyzing the standard curve of serially diluted cDNA. The amplification efficiency of the primers used in this study was from 90% to 110%. The relative abundance of a mRNA to 18S rRNA was calculated using the ΔΔCt method38. The expression levels of all mRNAs were presented as relative values to the average expression level of Mb mRNA in Stac3−/− muscles, which was normalized to 1.
Statistics
Student’s t test was used to determine the statistical significance of the differences between two groups. ANOVA followed by Tukey’s test was used to examine the statistical significance of differences between more than two groups. Before ANOVA or Student’s t test, homogeneity of variance between groups was analyzed using the F test. If unequal variances were detected, data were log-transformed. A difference was considered statistically significant when P < 0.05. All data were presented as mean ± standard error of the mean (SEM).
Additional Information
How to cite this article: Cong, X. et al. Defective excitation-contraction coupling is partially responsible for impaired contractility in hindlimb muscles of Stac3 knockout mice. Sci. Rep. 6, 26194; doi: 10.1038/srep26194 (2016).
References
Murray, L. M. et al. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 17, 949–962 (2008).
Wu, H. T., Xiong, W. C. & Mei, L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137, 1017–1033 (2010).
Al-Qusairi, L. et al. T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc. Natl. Acad. Sci. USA 106, 18763–18768 (2009).
Al-Qusairi, L. & Laporte, J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet. Muscle 1(26), 1–26 (2011).
O’Brien, R. O. et al. Exclusion of defects in the skeletal muscle specific regions of the DHPR alpha 1 subunit as frequent causes of malignant hyperthermia. J. Med. Genet. 32, 913–914 (1995).
Pietri-Rouxel, F. et al. DHPR alpha1S subunit controls skeletal muscle mass and morphogenesis. EMBO J. 29, 643–654 (2010).
Sutko, J. L. & Airey, J. A. Ryanodine receptor Ca2+ release channels: does diversity in form equal diversity in function? Physiol. Rev. 76, 1027–1071 (1996).
Capes, E. M., Loaiza, R. & Valdivia, H. H. Ryanodine receptors. Skelet. Muscle 1, 18 (2011).
Rebbeck, R. T. et al. Skeletal muscle excitation-contraction coupling: who are the dancing partners? Int. J. Biochem. Cell Biol. 48, 28–38 (2014).
Legha, W. et al. stac1 and stac2 genes define discrete and distinct subsets of dorsal root ganglia neurons. Gene Expr. Patterns 10, 368–375 (2010).
Nelson, B. R. et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 110, 11881–11886 (2013).
Reinholt, B. M., Ge, X., Cong, X., Gerrard, D. E. & Jiang, H. Stac3 is a novel regulator of skeletal muscle development in mice. PLoS One 8, e62760 (2013).
Horstick, E. J. et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 4, 1952 (2013).
Polster, A., Perni, S., Bichraoui, H. & Beam, K. G. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA 112, 602–606 (2015).
Zaharieva, I. T. et al. Whole exome sequencing in patients with congenital myopathies. Neuromuscul. Disord. 24, 895–895 (2014).
Bower, N. I. et al. Stac3 Is Required for Myotube Formation and Myogenic Differentiation in Vertebrate Skeletal Muscle. J. Biol. Chem. 287, 43936–43949 (2012).
Ge, X., Zhang, Y., Park, S., Cong, X. & Gerrard, D. E. Stac3 Inhibits Myoblast Differentiation into Myotubes. Plos One 9, e95926 (2014).
Zhang, Y., Cong, X., Wang, A. & Jiang, H. Identification of the STAC3 gene as a skeletal muscle-specifically expressed gene and a novel regulator of satellite cell differentiation in cattle. J. Anim. Sci. 92, 3284–3290 (2014).
Schiaffino, S. & Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 91, 1447–1531 (2011).
Rousseau, E., Ladine, J., Liu, Q. Y. & Meissner, G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch. Biochem. Biophys. 267, 75–86 (1988).
Allen, D. G. & Westerblad, H. The effects of caffeine on intracellular calcium, force and the rate of relaxation of mouse skeletal muscle. J. Physiol. 487 (Pt 2), 331–342 (1995).
Rossi, R., Bottinelli, R., Sorrentino, V. & Reggiani, C. Response to caffeine and ryanodine receptor isoforms in mouse skeletal muscles. Am. J. Physiol. Cell Physiol. 281, C585–594 (2001).
Fessenden, J. D. et al. Divergent functional properties of ryanodine receptor types 1 and 3 expressed in a myogenic cell line. Biophys. J. 79, 2509–2525 (2000).
Lanner, J. T., Georgiou, D. K., Joshi, A. D. & Hamilton, S. L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2, a003996 (2010).
Schiaffino, S. et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J. Myscle Res. Cell Motil. 10, 197–205 (1989).
Caiozzo, V. J., Haddad, F., Baker, M. J. & Baldwin, K. M. Influence of mechanical loading on myosin heavy-chain protein and mRNA isoform expression. J. Appl. Physiol. 80, 1503–1512 (1996).
Ohira, Y. et al. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol. 87, 1776–1785 (1999).
Trappe, S. et al. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J. Physiol. 557, 501–513 (2004).
Li, S. S. Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction. Biochem. J. 390, 641–653 (2005).
Saksela, K. & Permi, P. SH3 domain ligand binding: What’s the consensus and where’s the specificity? FEBS Lett. 586, 2609–2614 (2012).
Grange, R. W., Gainer, T. G., Marschner, K. M., Talmadge, R. J. & Stull, J. T. Fast-twitch skeletal muscles of dystrophic mouse pups are resistant to injury from acute mechanical stress. Am. J. Physiol. Cell Physiol. 283, C1090–1101 (2002).
Takeshima, H. et al. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature 369, 556–559 (1994).
Rando, T. A. & Blau, H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 (1994).
Pisaniello, A. et al. The block of ryanodine receptors selectively inhibits fetal myoblast differentiation. J. Cell Biol. 116, 1589–1597 (2003).
Bakker, A. J., Head, S. I., Williams, D. A. & Stephenson, D. G. Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J. Physiol. 460, 1–13 (1993).
Park, S., Scheffler, T. L. & Gerrard, D. E. Chronic high cytosolic calcium decreases AICAR-induced AMPK activity via calcium/calmodulin activated protein kinase II signaling cascade. Cell calcium 50, 73–83 (2011).
Suga, T. et al. Muscle fiber type-predominant promoter activity in lentiviral-mediated transgenic mouse. PLoS One 6, e16908 (2011).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408 (2001).
Author information
Authors and Affiliations
Contributions
X.C. performed all experiments, analyzed data, and drafted the manuscript; J.D. performed the muscle contraction experiment; R.W.G. designed the muscle contraction experiment, interpreted results, and revised the manuscript; H.J. conceived and designed the project, interpreted results, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Cong, X., Doering, J., Grange, R. et al. Defective excitation-contraction coupling is partially responsible for impaired contractility in hindlimb muscles of Stac3 knockout mice. Sci Rep 6, 26194 (2016). https://doi.org/10.1038/srep26194
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep26194
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.