Abstract
Elevated global temperatures and increased concentrations of carbon dioxide (CO2) in the atmosphere associated with climate change will exert profound effects on rice cropping systems, particularly on their greenhouse gas emitting potential. Incorporating biochar into paddy soil has been shown previously to reduce methane (CH4) emission from paddy rice under ambient temperature and CO2. We examined the ability of rice straw-derived biochar to reduce CH4 emission from paddy soil under elevated temperature and CO2 concentrations expected in the future. Adding biochar to paddy soil reduced CH4 emission under ambient conditions and significantly reduced emissions by 39.5% (ranging from 185.4 mg kg−1 dry weight soil, dws season−1 to 112.2 mg kg−1 dws season−1) under simultaneously elevated temperature and CO2. Reduced CH4 release was mainly attributable to the decreased activity of methanogens along with the increased CH4 oxidation activity and pmoA gene abundance of methanotrophs. Our findings highlight the valuable services of biochar amendment for CH4 control from paddy soil in a future that will be shaped by climate change.
Similar content being viewed by others
Introduction
Climate change is unequivocal and inevitable. Since the 1950 s, average global mean surface temperature has increased by 0.72 °C and it is projected to further increase by 1.5 to 4.5 °C by the end of this century1. Atmospheric carbon dioxide (CO2) has also risen to 391 ppm at 2011, which exceeded the pre-industrial levels by about 40%, and is predicted to increase to between 421 and 936 ppm by 21001. Projected increases in global temperature and atmospheric CO2 threaten future food security. It is well reported that crop yields will be influenced remarkably by interactions between elevated atmospheric CO2 and temperature2,3,4. Results of a meta-analysis on the response of rice yield to warming conditions (+0.8 °C to +6 °C) suggest that warming would significantly decrease yields by 14.6% for every 1 °C increase in temperature5.
Although many factors contribute to the warming trends observed, the main driver is generally attributed to increasing greenhouse gas (GHG) emissions. Methane (CH4) is the second most important GHG after CO2. It has over 25 times the global warming potential of CO2 over a 100-year forward prediction6 and is responsible for approximately 20% of the anthropogenic warming effect7. Rice cropping systems are considered to be among the major anthropogenic sources of CH48. Estimates of the annual contribution of CH4 emissions from paddy soils range from 31 to 112 Tg y−1, accounting for 9–19% of total global CH4 emissions8,9. Worse still, a huge number of farmers have been pouring fertilizers and rice straws into paddy soil, both of which lead to increased emissions of CH410,11,12 and thus aggravate climate warming.
Many studies have demonstrated that elevated atmospheric CO2 and temperature would further increase CH4 emission from paddy fields9,13,14,15. Meta-analyses of the effect of rising atmospheric CO2 and warming on CH4 emissions from rice paddies showed that increased CO2 levels of 460–780 ppm could stimulate CH4 emissions by over 40%5,13. However, reduced CH4 emissions from paddy soil have also been reported to occur with elevated temperature and CO216,17,18. Regardless of the positive or negative effect of climate change, CH4 emission control from paddy soil is of critical importance now and in the future.
Biochar is the charcoal product resulting from the thermal decomposition of organic materials under a limited oxygen supply (pyrolysis)19. Biochar is not a homogeneous product, rather its properties vary according to the organic materials (feedstock) pyrolyzed and the time and temperature of pyrolysis. Biochar is a popular soil amendment intended to improve soil fertility by increasing soil nutrient retention19,20 and increasing soil water holding capacity thus enhancing primary productivity21. Biochar amendment is also promoted as a means to sequester stabilized carbon (C) in soil22,23,24. Previous studies have shown that amending paddy soils with biochar derived from rice straw can significantly decrease CH4 emissions by more than 80% compared to corresponding controls25,26,27. Decreased CH4 emissions were attributed mainly to the effects of biochar on soil physicochemical factors and changes in microbial communities, particularly decreases in the abundance and activity of the methanogens that produce CH4 and increases in the abundance and activity of the methanotrophs that oxidize it26,27,28,29. However, it remains unknown whether biochar can be used effectively to reduce CH4 emissions from rice cropping systems under projected changes in global temperature and atmospheric CO2 concentrations.
We investigated the effects of rice straw-derived biochar on CH4 emission from paddy soil under elevated temperature and CO2 through a chamber-scaled experiment. To provide adequate evidence and reveal the potential mechanism, we determined soil biochemical variables, the abundances of 16 S rRNA genes of methanogens, the abundances of the particulate methane monooxygenase genes (pmoA), and rice plant growth and yield. Results of this study will provide solid evidence that rice straw-derived biochar is an effective soil amendment for reducing CH4 emissions from paddy soils under projected climate change.
Results
Rice plant growth
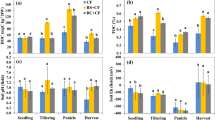
Paddy soil was either amended with biochar (BC treatments) or left unamended (CK treatments) and then planted with rice. Rice plants were grown under ambient (CK, BC) or elevated temperature (+3 °C, tCK, tBC), or elevated CO2 (700 ppm, cCK, cBC), or simultaneously elevated temperature and CO2 (+3 °C and 700 ppm, tcCK, tcBC). Elevated temperature alone (tCK) significantly (p < 0.05) reduced total and above-ground rice biomass respectively compared to the control (CK) (Fig. 1). Elevated CO2 alone (cCK) significantly (p < 0.05) promoted the total, above-ground and root biomass of rice plants grown than the corresponding control (CK). Biochar amendment under ambient (BC) and elevated CO2 (cBC) conditions increased the total and above-ground biomass of rice plants significantly (p < 0.05) compared to their corresponding controls (CK and cCK). Moreover, biochar addition in the simultaneously elevated temperature and CO2 system significantly (p < 0.05) increased the total and above-ground biomass of rice plants, respectively.
The values presented in the columns are mean ± standard deviations (n = 4). Different lowercase letters indicate significant differences between the eight treatments (p < 0.05). Rice plants were grown under ambient (CK, BC) or elevated temperature (tCK, tBC), or elevated CO2 (cCK, cBC), or simultaneously elevated temperature and CO2 (tcCK, tcBC). Paddy soil was either unamended (CK) or amended with biochar (BC) (2.5% w/w).
CH4 emission patterns
There was a similar trend of CH4 emission flux across all treatments during the overall rice growing season with the peak occurring at the heading stage (Fig. 2). The CH4 emission flux in tCK and cCK at the heading stage was much lower compared to that in CK, but higher in tcCK. Biochar amendment, to some extent, reduced the CH4 emission flux from paddy soil under all experimental conditions. The cumulative CH4 emissions from the paddy soils during the overall rice growing season showed remarkable differences among all treatments (Fig. 2). In contrast to CK, the cumulative CH4 emissions from tCK and cCK were significantly lower (p < 0.05). No significant difference in the cumulative CH4 emissions was observed between tcCK and CK. Biochar addition reduced CH4 emissions under ambient conditions remarkably (p < 0.05), ranging from 171.2 mg kg−1 dry weight soil, dws to 4.8 mg kg−1 dws. The addition of biochar either under elevated temperature or elevated CO2 had no significant impact on the cumulative CH4 emissions. Nevertheless, the application of biochar played a notable role in reducing the cumulative CH4 emissionsfrom paddy soil under simultaneously elevated temperature and CO2 conditions (p < 0.05). There was a significantly (p < 0.05) lower cumulative CH4 emissions from tcBC (112.2 mg kg−1 dws) than that from tcCK (185.4 mg kg−1 dws).
Seasonal variation of CH4 emission flux under different temperature and CO2 concentration with and without BC amendment (a) ambient; (b) elevated temperature; (c) elevated CO2; (d) elevated temperature and CO2 simultaneously) during the whole rice growing season, and the cumulative CH4 emissions in different treatments. The total cumulative methane emissions from 0 to 135 days are shown in Supplementary Fig. S1 in the “Supplementary Information”. Treatment designations of the cumulative CH4 emissions are showed below each column and different letters indicate significant differences between the eight treatments (p < 0.05). Treatment legend is given in Fig. 1.
Soil methanogenic and CH4 oxidation activity
Variations of methanogenic and CH4 oxidation activity in rhizosphere soils at the tillering and the heading stages are presented in Fig. 3. Although there were no significant differences in soil methanogenic activity among CK, tCK, cCK and tcCK at the tilling stage, significantly (p < 0.05) lower activity in tCK (10.7 μmol CH4 kg−1 dws h−1) and cCK (11.2 μmol CH4 kg−1 dws h−1) was detected at the heading stage as compared with that in CK (12.1 μmol CH4 kg−1 dws h−1) (Fig. 3a). The addition of biochar resulted in a significant reduction of methanogenic activity both at the tillering and heading stage under all conditions tested (p < 0.05), with the exception of the elevated CO2 at the heading phase (Fig. 3a). The soil CH4 oxidation activity in CK was much higher at the tilling stage in comparison with any other treatments (Fig. 3b). At the heading stage, despite no significant differences in the soil CH4 oxidation activity of tCK, cCK or tcCK in contrast to that of CK, biochar addition exerted a marked influence on the soil CH4 oxidation potential under different conditions. The CH4 oxidation activity in the BC and tcBC treatments was increased by 79.0% and 162.3% as compared to their corresponding controls (CK, tcCK) (p < 0.05) (Fig. 3b).
Methanogenic (a) and CH4 oxidation activity (b) in the rhizosphere soil at the tillering and heading stages in different treatments. Different letters indicate significant differences between the eight treatments at the same rice stage (p < 0.05). Treatment legend is given in Fig. 1.
Abundances of methanogenic archaeal 16 S rRNA genes and methanotrophic bacterial pmoA genes
Responses of the copy numbers of methanogenic archaeal 16 S rRNA genes in paddy soil to biochar addition, elevated temperature, elevated CO2, and simultaneously elevated temperature and CO2 are shown in Fig. 4a. At the tillering stage, methanogenic archaeal 16 S rRNA genes abundance from tCK, cCK or tcCK was significantly (p < 0.05) higher than that from CK. Biochar amendment significantly (p < 0.05) increased the copy numbers of 16 S rRNA genes for methanogens at the tilling stage, with the exception of elevated CO2. There were no significant differences in methanogenic archaeal 16 S rRNA genes abundance among paddy soils between BC addition and non-amended treatments at the heading stage.
Abundance of methanogenic 16 S rRNA genes (a) and methanotrophic pmoA genes (b) in the rhizosphere soil at the tillering and heading stages in different treatments. Different letters indicate significant differences between the eight treatments at the same rice stage (p < 0.05). Treatment legend is given in Fig. 1.
Changes in the copies of methanotrophic pmoA gene in different paddy soils are showed in Fig. 4b. At the tillering stage, only elevated CO2 resulted in significant reduction of soil methanotrophic pmoA gene abundance (p < 0.05). Biochar amendment significantly (p < 0.05) increased the abundance of methanotrophic pmoA gene under ambient, elevated temperature, and elevated CO2 condition. At the heading stage, copy numbers of methanotrophic pmoA gene from tCK and cCK were significantly (p < 0.05) higher than that from CK. Compared with their corresponding control, biochar addition led to a significant increase in the methanotrophic pmoA gene abundance (p < 0.05) except under the elevated CO2 condition. In contrast, the copy number of methanotrophic pmoA gene from tcBC (1.30 × 105 copies g−1 dws) was significantly (p < 0.05) lower than that from BC (3.38 × 105 copies g−1 dws).
Discussion
This is the first study to investigate the role of biochar in mitigating CH4 emission from paddy soil under elevated temperature and CO2 condition. It is imperative to better understand the emission of CH4 influenced by different temperature and CO2 concentration, as well as to address and elucidate the mechanistic effects of biochar amendment on CH4 emission from paddy soil under the predicted climate change.
In this study, variations of temperature and CO2 concentration had different influences on CH4 emissions during the rice growing season (Fig. 2). Our results revealed that CH4 emissions were reduced under elevated temperature or elevated CO2 alone, where cumulative CH4 emissions were reduced by 70.9% and 54.4%, respectively, compared with those treatments under ambient temperature and CO2. However, elevating temperature and CO2 simultaneously did not exert any significant effects on the cumulative CH4 emissions, rather increased them by 8.3%. The different responses of CH4 emission to environmental factors might be due to the variations in rice plant growth and subsequent effects on CH4 production. Elevated temperature significantly reduced the total and above-ground biomass of rice plants (Fig. 1). Baker et al.30 also reported the reduced rice yield under elevated temperatures. Since 80–90% of CH4 released into the atmosphere was rice plant-mediated through the well-developed aerenchyma31,32, inhibited rice growth caused by elevated temperature can partially reduce the emissions of CH4 through rice plants17. The optimal temperature of most mesophilic methanogens is about 35 °C, with methanogenic activity showing a decreasing trend when above this temperature33,34,35. This is one of the causes that in our study, elevated temperature significantly decreased soil methanogenic activity at rice heading stage (Fig. 3a), and then weakened CH4 emissions. On the other hand, CO2 enrichment strikingly promoted the biomass of rice plants (Fig. 1), which could bring more available oxygen to the rhizosphere soil. These impacts led to the decreased CH4 emissions by depressing methanogenesis and accelerating the soil methanotrophic growth (Figs 3a and 4b). It was well supported by the results of Schrope et al.17 and Inubushi et al.36, who reported that CO2 enrichment reduced CH4 production and promoted CH4 oxidation through the benefit of increased oxygen delivery through rice plants. In addition, rice biomass was similar in the control (CK) and in soil with simultaneously elevated temperature and CO2 (tcCK) (Fig. 1). One possibility would be that CO2 enrichment weakened the negative effects of elevated temperature on rice plants growth, providing a certain amount of substrates for CH4 production. However, the observed results could not come to such an effect. Further research is required before conclusions can arrive. Increases in atmospheric temperature and CO2 concentration turned out to be driving forces for CH4 emission from paddy soil (Fig. 2). Thus, CH4 control from paddy soil needs to be paid more attention in the future due to the predicted rise of both temperature und CO2.
Our results confirmed that CH4 emissions in paddy soil under ambient condition were reduced significantly by the application of biochar. Biochar amendment did significantly reduce the cumulative CH4 emissions by 97.2% compared to the control (Fig. 2a). The addition of biochar under ambient conditions attenuated the methanogenic activity remarkably at both the tillering and the heading stages, and improved methanotrophic pmoA gene abundance and potential activity at the heading stage (Figs 3a and 4b). These findings are similar to the results of many previous studies, which reported that biochar amendment could make the rhizosphere soil favorable for methanotrophs but unfavorable for methanogens25,26,27. Therefore, it was the stimulated methanotrophic activity and inhibited methanogenic activity caused by biochar application that led to the declined CH4 emissions in ambient system. These results verify that the application of rice straw biochar not only stimulate rice plant productivity37,38, but also suppress CH4 emissions from paddy soil.
Interestingly, biochar amendment also significantly decreased CH4 emission from paddy soil under simultaneously elevated temperature and CO2 condition. Compared to the corresponding control (tcCK), the cumulative CH4 emissions in tcBC were reduced by 39.5% (Fig. 2d). Based on the role of biochar in decreasing CH4 emission under ambient environmental conditions, we hypothesized that biochar addition would have notable influence on CH4 production and oxidation under the combined condition. As was expected, methanogenic activity decreased and CH4 oxidation potential increased when biochar was applied in simultaneously elevated temperature and CO2 system at the heading stage (Fig. 3). Spearman correlations and the correlation circle were calculated to compare the CH4 emission rates with biochemical and microbial gene data. We observed a significantly positive correlation between CH4 emission rate and methanogenic activity (rho = 0.500, p < 0.01) and CH4 oxidation activity (rho = 0.533, p < 0.01) (Fig. 5; Supplementary Table S1). This confirms the important role that methanogenic activity and CH4 oxidation activity have in controlling CH4 emission from paddy soil.
It is well-known that variations of biochemical and microbial parameters can cause different CH4 fluxes by influencing CH4 production and oxidation processes. Soil CH4 production is affected by the availability of labile carbon substrates39. Soil dissolved organic carbon (DOC) contributes to a great deal of carbon sources for methanogenic growth40. A decrease of soil DOC content from tcBC compared to tcCK at the heading time could explain the reduced CH4 emissions observed due to the decreased methanogenic activity (Supplementary Fig. S2a). As is proved, absorption of soil organic carbon onto biochar particles may have reduced substrate availability for CH4 production40. Liu et al.25 also illustrated that biochar amendment significantly reduced the soil methanogenic activity and CH4 emissions from paddy soil, mainly benefiting from the lack of substrate availability for methanogens. Meanwhile, we observed a greater concentration of microbial biomass carbon (MBC) in tcBC at the heading time, which suggests a faster succession of microorganisms by consuming easily available soil organic carbon (Supplementary Fig. S2b). This may then have retarded CH4 production and methanogenic activity by forming a more recalcitrant organic carbon pool41. Moreover, biochar addition under simultaneously elevated temperature and CO2 condition significantly promoted rice plants growth by improving the total and above-ground biomass (Fig. 1). The stimulated rice plants could bring more oxygen to the aerenchyma tissues of rhizosphere28,42, thus inhibiting methanogenic activity and increasing methanotrophic activities (Fig. 3). Some studies also demonstrated that the high porosity and large surface area of biochar may enhance the adsorption of CH425,28, providing substrates for methanotrophs and thus reducing CH4 emissions.
Furthermore, a significantly positive correlation of CH4 oxidation activity and soil water content (rho = 0.542, p < 0.01), and pH value (rho = 0.439, p < 0.05) indicated the important role of soil moisture and pH in affecting soil CH4 oxidation (Fig. 5; Supplementary Table S1). Soil water content increased considerably in tcBC in comparison to that in tcCK at the heading time (Supplementary Fig. S3). This broadened the optimum range of water content for methanotrophy43,44, and then stimulated CH4 oxidation activity (Fig. 3b). Studies have noted that the highly porous structure of biochar could increase water holding capacity, and thus increase CH4 oxidation by restricting soil moisture fluctuations40,45,46. Schnell et al.47 also reported that CH4 uptake rates would increase with increasing water content. Moreover, methanotrophs are usually sensitive to the fluctuation of soil pH values39. Our results showed that biochar amendment could significantly increase the pH values ranging from 5.55 ± 0.10–5.71 ± 0.01 under simultaneously elevated temperature and CO2 condition at the heading stage (Supplementary Fig. S4). The increased pH was favorable for methanotrophs (the optimal pH value is 6.0–7.0)48, promoting methanotrophic potential (Fig. 3b).
The CH4 emission rate displayed significant negative correlations with the abundance of methanotrophic pmoA gene (rho = −0.558, p < 0.05) (Supplementary Table S1). In simultaneously elevated temperature and CO2 system, biochar addition improved the copy numbers of methanotrophic pmoA gene significantly, stimulating methanotrophic growth at the heading stage (Fig. 4b). As a result of the apparently promoted methanotrophic pmoA gene abundance, CH4 oxidation activity was enhanced greatly (Fig. 3b). The observed increase in the methanotrophic pmoA gene abundance might benefit from the abundant CH4 as the only C substrate for methanotrophs26, as well as from the oxygen condition40 and living habitat49 supplied by biochar addition. Our results were similar to Feng et al.26, who also showed that biochar addition could significantly promote methanotrophic growth with the increased abundances of pmoA gene in paddy soil, explaining the reduced CH4 emissions. This study demonstrated that biochar incorporation resulted in the significant increases in rice biomass, pH, moisture and methanotrophic pmoA gene abundance, and decrease in labile organic carbon. These variations would inhibit CH4 production and promote CH4 oxidation, and thereby lower CH4 emission under the combined condition.
The community structure and composition of methanogens and methanotrophs could exert great effects on CH4 production and oxidation33,39. Potential activity and the copy numbers of key genes observed in this study could not represent the structure and metabolism difference of methanogens and methanotrophs communities, during the heading time, although an evidently higher methanogenic activity and less stimulatory methanotrophic growth in tcBC statistically explained parts of the difference of CH4 emission under ambient and the combined conditions (Figs 3a and 4b). Thus, further research to assess the environmental functions and structures of methanogens and methanotrophs responsible in different systems is highly desirable. These findings suggested that although the effect of biochar addition on CH4 mitigation was weakened, it could also assist in making paddy soil a great CH4 sink under the combined condition (Fig. 2). It will provide valuable rice ecosystem services, which become increasingly important for CH4 control from paddy soil in the projected warming climate.
In conclusion, our findings not only confirmed that biochar amendment decreased CH4 emissions from paddy soil under ambient condition, but also, for the first time, demonstrated that biochar addition did significantly reduce CH4 emissions by 39.5% from paddy soil under simultaneously elevated temperature and CO2 conditions. The reduced CH4 emissions were mainly due to the reduced CH4 production and release as a result of the inhibition to methanogens and promotion to methanotrophs, which were caused by changes in biochemical and microbial characteristics with biochar addition. These results imply that biochar amendment into paddy soil can be an effective strategy for CH4 mitigation under the elevated temperature and CO2 condition. In addition, it is relevant as our earth is predicted to become warmer and highlights the value of biochar in assisting with slowing down the greenhouse effect caused by CH4 emission from paddy soil. Moreover, it will pave a way for human beings to curb the inevitably warming climate by adopting biochar to reduce the anthropogenic CH4 emissions. Nevertheless, the variation of structure and composition in functional methanogens and methanotrophs communities is still unclear. Further research about the effects of biochar amendment on CH4 mitigation from paddy soil needs to elucidate the microbial mechanism of CH4 release under simultaneously elevated temperature and CO2 condition.
Methods
Soil and Biochar
Paddy soil (0–15 cm) used in this study was collected from a traditional, representative rice field in the Yuhang District (119.5°E, 30.2°N), Hangzhou, Zhejiang Province, China. The soil was air-dried, ground and sieved through a 2 mm mesh screen prior to use. Rice straw-derived biochar was produced through slow pyrolysis of rice straw at 500 °C with a mean residence time of 3 h. The pH, electrical conductivity (EC), total carbon (TC) total nitrogen (TN), bulk density and cation exchange capacity (CEC) of the soil and biochar were measured and are given in Table 1.
Microcosms
Microcosm studies were carried out at the Agricultural Experiment Station of Zhejiang University, Hangzhou, China, in a growth chamber system consisting of four 1.85 × 0.86 × 1.95 m (length × width × height) growth chambers, which could control the interior CO2 concentration, air temperature, lighting time and relative humidity automatically.
Biochar amendment treatments (BC) were applied at a rate of 2.5% (w/w) and mixed homogenously into the soil. A control treatment (CK) without biochar addition was established for comparison. Air temperature was set to approximately follow the ambient air temperature of April to August in Hangzhou city, China for the control treatments and +3 °C for the elevated temperature treatments (Supplementary Table S2). Lighting time was set near to the ambient condition (Supplementary Table S2). The ambient CO2 concentration was kept at 390 ± 10 ppm and the elevated CO2 was set at 700 ± 10 ppm. The eight treatment combinations were: CK and BC at ambient temperature and ambient CO2; the two soil treatments under elevated temperature and ambient CO2 (tCK and tBC); the two soil treatments under ambient temperature and elevated CO2 (cCK and cBC); and the two soil treatments under elevated temperature and elevated CO2 (tcCK and tcBC). Each treatment was replicated four times. Cylindrical polyvinylchloride pots (30 cm high and 20 cm inner diameter), equipped with a water tank (5 cm high) at the top for containing the gas sampling chamber and water seals, were filled with a 5 kg soil (CK) or a total 5 kg mass of soil and biochar.
All treatments were pre-incubated by flooding the soil with deionized water by 2–3 cm above the soil and placing them into the growth chambers for ten days before rice transplanting (Oryza sativa L). The pots were then randomly arranged at regular intervals to take account of subtle differences in light, temperature and CO2 within each chamber. Flooded conditions were maintained throughout the main rice growing stages, and then drained when the rice grew during the tillering stage (30 days after transplanting, 30 DAT). The draining ended at 40 DAT and the flooded conditions would stay thereafter. The basal fertilizers, urea-[CO(NH2)2] (60 mg N kg−1), and phosphate and potassic fertilizer-KH2PO4 and KCl (100 mg P2O5 kg−1 and 100 mg K2O kg−1) were applied and mixed homogenously at the pre-incubation time of treatments in chambers. Nitrogen fertilizer (urea) was top-dressed at early tillering phase (18 DAT) (45 mg N kg−1) and at the early heading phase (82 DAT) (45 mg N kg−1). Relative humidity was kept at 65 ± 2%.
CH4 flux measurement
The CH4 flux was determined by the closed chamber method50. Details about the gas sampling procedure, structure of chamber and the measurement of CH4 concentration are presented in the “Supplementary Information”.
Physicochemical analysis
During the pivotal rice growth phases - tillering (23 DAT) and heading (90 DAT) - rhizosphere soil samples were collected. Samples for molecular analysis and physicochemical measurements were kept at −70 °C and 4 °C, respectively.
Microbial biomass C (MBC), determined by the fumigation-extraction method51, and soil dissolved organic C (DOC), were both determined by an automated total organic C (TOC) Analyzer (Multi N/C 2100, Jena, Germany). Details about the measurement procedure of MBC and DOC are presented in the “Supplementary Information”. Soil pH was measured after suspending soil in water (1:2.5 w/w).
The methanogenic activity of paddy soils was measured in triplicate, by using fresh soil samples (10 g) mixed with 0.2 mmol oxygen-free sterile glucose solution in 100 ml serum bottle. The bottles were flushed with O2-free N2 for 3 min sealed with butyl rubber lids and aluminum crowns, and incubated at 28 °C for 24 h. CH4 contained in the headspace of the serum bottle was determined by gas chromatography. The methanogenic activity was expressed as micromoles of CH4 per kilogram of dry weight soil (dws) per hour52.
Soil CH4 oxidation activity was analyzed according to the method applied by Hanson53. Triplicate fresh soil samples (10 g) were placed in 100 mL serum bottles, sealed with butyl rubber lids and aluminum crowns. Each bottle was then injected with 5 mL of highly pure CH4 and incubated without light at 28 °C for 8 h. Empty but CH4-amended bottles were set as controls. CH4 in the headspace of the serum bottles was measured by using gas chromatography. CH4 oxidation activity was expressed as consumed micromoles of CH4 per kilogram of dry weight soil per hour54.
Rice biomass. After the rice grains were harvested, the above-ground parts of the rice plants were cut above 2 cm from the soil surface and removed. The rice roots in the pot were gently and thoroughly washed with water. The aboveground parts and roots of the rice plants were then oven-dried at 80 °C for 72 h to measure dry matter weight55.
DNA extraction and quantification of functional microbial communities
For each sample at the rice tillering and heading stages, 0.5 g soil was used for DNA extraction with a FastDNA® SPIN Kit for soil (MP Biomedical, LLC, OH, USA) according to the manufacturer’s instructions. The extracted soil DNA was then dissolved in 80 mL tris-EDTA (TE) buffer, stored at −20 °C until further use.
Quantitative PCR (qPCR) was used to estimate the abundances of methanogenic archaeal 16 S rRNA genes and pmoA, the functional gene encoding the key enzyme involved in methane oxidation (particulate methane monooxygenase) using the primer pairs 0357 F/0691 R56 and A189f/mb661r57, respectively. The quantification was based on the intensity of SYBR Green dye fluorescence. The PCR reaction mixture consisted of 2 μL of template DNA, 0.1 μmol L−1 of each primer, 1 × SYBR Premix EX Taq (Perfect Real Time) premix reagent and ultrapure DNase/RNase-free water (ddH2O) to a final volume of 20 μL. The primers and thermal cycling used for each reaction are given in “(Supplementary Table S3)”. Reactions were performed in triplicate in a Bio-Rad CFX1000 Thermal Cycler, and ddH2O was used as a negative control template. The gene copy numbers were calculated according to the method of Wang et al.58. Standard curves were obtained using purified plasmids of a 16 S rRNA gene of methanogens and pmoA gene clones.
Statistical analyses
Treatment means and standard deviation (SD) were calculated. SPSS 20.0 statistical software package (SPSS Inc., Chicago, IL, USA) was used to determine significant difference among different treatments and correlation analysis. Significance of differences between groups was determined by analysis of variance LSD test. p-value < 0.05 was considered statistically significant. The correlation circle between CH4 emissions and other indexes was analyzed by the MFA (Multiple Factor Analysis) method through R software at http://www.R-project.org (R Development Core Team, 2014) from where it can be freely downloaded.
Additional Information
How to cite this article: Han, X. et al. Mitigating methane emission from paddy soil with rice-straw biochar amendment under projected climate change. Sci. Rep. 6, 24731; doi: 10.1038/srep24731 (2016).
References
Alexander, L. V. T. et al. Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis. Working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. (eds Stocker, T., et al. ) 3–29 (Cambridge Univ. Press, 2013).
Baker, J. T., Allen, L. H. & Boote, K. J. Response of rice to carbon-dioxide and temperature. Agr. Forest meteorol. 60, 153–166 (1992).
Peng, S. B. et al. Rice yields decline with higher night temperature from global warming. Proc. Natl Acad. Sci. USA 101, 9971–9975 (2004).
Ray, D. K., Gerber, J. S., MacDonald, G. K. & West, P. C. Climate variation explains a third of global crop yield variability. Nat. Commun. 6, 5989 (2015).
van Groenigen, K. J., van Kessel, C. & Hungate, B. A. Increased greenhouse-gas intensity of rice production under future atmospheric conditions. Nat. Clim. Chang 3, 288–291 (2013).
Shindell, D. T. et al. Improved attribution of climate forcing to emissions. Science 326, 716–718 (2009).
Cheng, W., Yagi, K., Xu, H., Sakai, H. & Kobayashi, K. Influence of elevated concentrations of atmospheric CO2 on CH4 and CO2 entrapped in rice-paddy soil. Chem. Geol. 218, 15–24 (2005).
Forster, P. et al. Changes in atmospheric constituents and in radiative forcing. In: Climate Change 2007: The Physical Science Basis. Working group I contribution to the fourth assessment report of the intergovernmental panel on climate change. (eds Solomon, S., et al. ) Ch. 2, 129–234 (Cambridge Univ. Press, 2007).
Tokida, T. et al. Methane and soil CO2 production from current-season photosynthates in a rice paddy exposed to elevated CO2 concentration and soil temperature. Glob. Change Biol. 17, 3327–3337 (2011).
Qiu, J. China cuts methane emissions from rice fields. Nature 18 (2009).
Ye, R. Z., Doane, T. A., Morris, J. & Horwath, W. R. The effect of rice straw on the priming of soil organic matter and methane production in peat soils. Soil Biol. Biochem. 81, 98–107 (2015).
Zhang, G. et al. Effect of rice straw application on stable carbon isotopes, methanogenic pathway, and fraction of CH4 oxidized in a continuously flooded rice field in winter season. Soil Biol. Biochem. 84, 75–82 (2015).
van Groenigen, K. J., Osenberg, C. W. & Hungate, B. A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2 . Nature 475, 214–216 (2011).
Cheng, W., Yagi, K., Sakai, H. & Kobayashi, K. Effects of elevated atmospheric CO2 concentrations on CH4 and N2O emission from rice soil: an experiment in controlled-environment chambers. Biogeochem. 77, 351–373 (2006).
Gaihre, Y. K., Wassmann, R. & Villegas-Pangga, G. Impact of elevated temperatures on greenhouse gas emissions in rice systems: interaction with straw incorporation studied in a growth chamber experiment. Plant Soil 373, 857–875 (2013).
Sass, R., Fisher, F., Turner, F. & Jund, M. Methane emission from rice fields as influenced by solar radiation, temperature, and straw incorporation. Global Biogeochem. Cycles 5, 335–350 (1991).
Schrope, M., Chanton, J., Allen, L. & Baker, J. Effect of CO2 enrichment and elevated temperature on methane emissions from rice, Oryza sativa. Glob. Change Biol. 5, 587–599 (1999).
Dijkstra, F. A. et al. Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: evidence from field experiments. Front. Ecol. Environ. 10, 520–527 (2012).
Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 5, 381–387 (2007).
Lehmann, J. A handful of carbon. Nature 447, 143–144 (2007).
Woolf, D., Amonette, J. E., Street-Perrott, F. A., Lehmann, J. & Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 1, 56 (2010).
Sohi, S. P. Carbon storage with benefits. Science 338, 1034–1035 (2012).
Marris, E. Putting the carbon back: Black is the new green. Nature 442, 624–626 (2006).
Lehmann, J., Czimczik, C., Laird, D. & Sohi, S. Stability of biochar in soil. In: Biochar For Environmental Management: Science And Technology. 1nd edn (eds Joseph, S. & Lehmann, J. ) Ch. 11, 183–206 (Earthscan, 2009).
Liu, Y. et al. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediments 11, 930–939 (2011).
Feng, Y., Xu, Y., Yu, Y., Xie, Z. & Lin, X. Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol. Biochem. 46, 80–88 (2012).
Khan, S., Chao, C., Waqas, M., Arp, H. P. H. & Zhu, Y. Sewage sludge biochar influence upon rice (Oryza sativa L) yield, metal bioaccumulation and greenhouse gas emissions from acidic paddy soil. Environ. Sci. Technol. 47, 8624–8632 (2013).
Dong, D. et al. Responses of methane emissions and rice yield to applications of biochar and straw in a paddy field. J. Soils Sediments 13, 1450–1460 (2013).
Zhao, X., Wang, J., Wang, S. & Xing, G. Successive straw biochar application as a strategy to sequester carbon and improve fertility: A pot experiment with two rice/wheat rotations in paddy soil. Plant Soil 378, 279–294 (2014).
Baker, J. T. et al. Carbon-dioxide and temperature effects on rice (Oryza-sativa L, CV IR 72). Soil Crop Sci. Soc. FL. 53, 90–97 (1994).
Wassmann, R. et al. Rennenberg, H., Fluxes and pools of methane in wetland rice soils with varying organic inputs. Environ. Monit. Assess. 42, 163–173 (1996).
Lou, Y. et al. CH4 emission with differences in atmospheric CO2 enrichment and rice cultivars in a Japanese paddy soil. Glob. Change Biol. 14, 2678–2687 (2008).
Oremland, R. S. Biogeochemistry of methanogenic bacteria. In: Biology Of Anaerobic Microorganisms. 1nd edn (eds Zehnder, A. J. ) 641–705 (Wiley-InterScience, 1988).
Parashar, D., Gupta, P. K., Rai, J., Sharma, R. & Singh, N. Effect of soil temperature on methane emission from paddy fields. Chemosphere 26, 247–250 (1993).
Cicerone, R. J. & Oremland, R. S. Biogeochemical aspects of atmospheric methane. Global Biogeochem. Cycles 2, 299–327 (1988).
Inubushi, K. et al. Effects of free-air CO2 enrichment (FACE) on CH4 emission from a rice paddy field. Glob. Change Biol. 9, 1458–1464 (2003).
Jeffery, S., Verheijen, F., Van Der Velde, M. & Bastos, A. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 144, 175–187 (2011).
Biederman, L. A. & Harpole, W. S. Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5, 202–214 (2013).
Conrad, R. Microbial ecology of methanogens and methanotrophs. Advan. Agron. 96, 1–63 (2007).
van Zwieten, L. et al. Biochar and emissions of non-CO2 greenhouse gases from soil. In: Biochar For Environmental Management: Science And Technology. 1nd edn (eds Joseph, S. & Lehmann, J. ) Ch. 13, 227–249 (Earthscan, 2009).
Yu, L., Tang, J., Zhang, R., Wu, Q. & Gong, M. Effects of biochar application on soil methane emission at different soil moisture levels. Biol. Fert. Soils 49, 119–128 (2013).
Watanabe, I., Hashimoto, T. & Shimoyama, A. Methane-oxidizing activities and methanotrophic populations associated with wetland rice plants. Biol. Fert. Soils 24, 261–265 (1997).
Abel, S. et al. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202, 183–191 (2013).
Cornelissen, G. et al. Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agronomy 3, 256–274 (2013).
Kammann, C., Grünhage, L., Jäger, H.-J. & Wachinger, G. Methane fluxes from differentially managed grassland study plots: the important role of CH4 oxidation in grassland with a high potential for CH4 production. Environ. Pollut. 115, 261–273 (2001).
Karhu, K., Mattila, T., Bergstrom, I. & Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity-Results from a short-term pilot field study. Agric. Ecosyst. Environ. 140, 309–313 (2011).
Schnell, S. & King, G. Responses of methanotrophic activity in soils and cultures to water stress. Appl. Environ. Microbiol. 62, 3203–3209 (1996).
Hanson, R. S. & Hanson, T. E. Methanotrophic bacteria. Microbiol. Reviews 60, 439–471 (1996).
Pietikäinen, J., Kiikkilä, O. & Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 89, 231–242 (2000).
Cai, Z. et al. Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant Soil 196, 7–14 (1997).
Vance, E., Brookes, P. & Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
Liu, W. et al. Transgenic Bt rice does not affect enzyme activities and microbial composition in the rhizosphere during crop development. Soil Biol. Biochem. 40, 475–486 (2008).
Hanson, R. S. Ecology of methylotrophic bacteria. In: Techniques in Microbial Ecology. 1nd edn (eds Burlage, R. S. et al. ) Ch. 6, 137–162 (Oxford Univ. Press, 1998).
Wang, Y. et al. Methane oxidation activity and bacterial community composition in a simulated landfill cover soil is influenced by the growth of Chenopodium album L. Soil Biol. Biochem. 40, 2452–2459 (2008).
Tokida, T. et al. The contribution of entrapped gas bubbles to the soil methane pool and their role in methane emission from rice paddy soil in free-air [CO2] enrichment and soil warming experiments. Plant Soil 364, 131–143 (2013).
Watanabe, T., Asakawa, S., Nakamura, A., Nagaoka, K. & Kimura, M. DGGE method for analyzing 16 S rDNA of methanogenic archaeal community in paddy field soil. FEMS Microbiol. Lett. 232, 153–163 (2004).
Lüke, C. et al. Macroecology of methane-oxidizing bacteria: the β-diversity of pmoA genotypes in tropical and subtropical rice paddies. Environ. Microbiol. 16, 72–83 (2014).
Wang, C. et al. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 47, 7341–7349 (2013).
Acknowledgements
This research was supported by the National Key Technology R&D Program (2015BAC02B01), the National Natural Science Foundation of China (41571241 and 41271247), and the Natural Science Foundation of Zhejiang Province (LZ15D030001).
Author information
Authors and Affiliations
Contributions
W.W., X.S. and X.H. designed the study, X.H. and X.S. performed the experiment, W.W., X.H. and C.W. analyzed the data, and X.H. wrote the paper. J. E.T. reviewed the manuscript and gave some comments. All co-authors participated in analyses and discussions and revised the manuscript. X.H. and X. S. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Han, X., Sun, X., Wang, C. et al. Mitigating methane emission from paddy soil with rice-straw biochar amendment under projected climate change. Sci Rep 6, 24731 (2016). https://doi.org/10.1038/srep24731
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24731
This article is cited by
-
A study on GHG emission assessment in agricultural areas in Sri Lanka: the case of Mahaweli H agricultural region
Environmental Science and Pollution Research (2023)
-
Contrasting emissions of carbon-based greenhouse gases from two paddy soils under submerged conditions as affected by biochar addition
Environmental Earth Sciences (2023)
-
Biochar Amendment Promoted the Maize Growth and Changed Bacterial Community Assembly in a Phenanthrene-Contaminated Soil
Journal of Soil Science and Plant Nutrition (2023)
-
Methane emissions and rice yield in a paddy soil: the effect of biochar and polystyrene microplastics interaction
Paddy and Water Environment (2023)
-
Effects of elevated CO2 concentration on CH4 and N2O emissions from paddy fields: A meta-analysis
Science China Earth Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.