Abstract
Ototoxic drugs, such as platinum-based chemotherapeutics, often lead to permanent hearing loss through apoptosis of neuroepithelial hair cells and afferent neurons of the cochlea. There is no approved therapy for preventing or reversing this process. Our previous studies identified a G protein-coupled receptor (GPCR), S1P2, as a potential mediator of otoprotection. We therefore sought to identify a pharmacological approach to prevent cochlear degeneration via activation of S1P2. The cochleae of S1pr2−/− knockout mice were evaluated for accumulation of reactive oxygen species (ROS) with a nitro blue tetrazolium (NBT) assay. This showed that loss of S1P2 results in accumulation of ROS that precedes progressive cochlear degeneration as previously reported. These findings were supported by in vitro cell-based assays to evaluate cell viability, induction of apoptosis and accumulation of ROS following activation of S1P2 in the presence of cisplatin. We show for the first time, that activation of S1P2 with a selective receptor agonist increases cell viability and reduces cisplatin-mediated cell death by reducing ROS. Cumulatively, these results suggest that S1P2 may serve as a therapeutic target for attenuating cisplatin-mediated ototoxicity.
Similar content being viewed by others
Introduction
Hair cells of the cochlea are specialized neuroepithelial cells required for the transduction of vibrational force into the perception of hearing. In mammals, these post-mitotic, terminally differentiated cells are fully developed shortly after birth, thus they do not have the capacity to regenerate if they are lost. There are 3 major causes of acquired (non-hereditary) sensorineural hearing loss: noise exposure, ototoxicity and age. These causes account for about half of the estimated 700 million cases of debilitating hearing loss worldwide1. Major classes of ototoxic drugs include chemotherapeutics (cisplatin), aminoglycoside antibiotics (kanamycin) and loop diuretics (furosemide). While the molecular events leading to ototoxicity are complex, there is evidence that they are, at least in part, mediated by toxic accumulation of reactive oxygen species (ROS). The NOX family of NADPH oxidases (NOX1-NOX5 and DUOX1–2) is a major source of endogenous ROS formation2. Its members are multi-subunit, membrane-associated enzymatic structures with complex regulatory machinery. One family member, NOX3, is highly and selectively expressed in the inner ear, with little detectable expression in other tissues3.
Despite information regarding the molecular events underlying cochlear degeneration, there are currently no therapies that can prevent or reverse this process. This is due in large part to the absence of pharmacologically tractable molecular targets that regulate cochlear viability. Previous work by our group and others provide evidence that S1P2, a G protein-coupled receptor (GPCR) that mediates the effects of sphingosine 1-phosphate (S1P), may represent such a target.
S1P is a bioactive lipid signalling molecule that is known to act as a potent extracellular ligand for a family of five cognate GPCRs, S1P1–S1P54. These receptors have distinct but overlapping patterns of expression and are known to be important activators of many cellular processes, such as cell proliferation, cell death, cytoskeletal rearrangement, migration/motility and differentiation5,6. Notably, receptor-mediated S1P signalling has been shown to affect the production of ROS in the heart7, blood vessels8, fibroblasts9 and hematopoietic progenitor cells10.
Many of the biomedically relevant roles of S1P receptors have been elucidated with the study of genetically engineered knockout mice. These studies have shown that S1P signalling is essential for a number of processes including vascular maturation11, lymphocyte trafficking12, epithelial sheet migration13, B cell regulation14, egress of natural killer cells15 and mechanisms underlying the multiple sclerosis drug known as fingolimod (Gilenya)16,17,18. Recently, it was shown that S1P2 knockout mice uniformly exhibit a progressive loss of inner ear function, resulting in profound deafness and vestibular dysfunction, demonstrating that S1P2 activity is necessary for cochlear viability19,20,21 that was a ligand-dependent process since loss of S1P transporter gene Spns2 phenocopies S1P2 loss22. Cochlear degeneration was associated with early vascular defects that likely alter cochlear perfusion pressure and disrupt electrochemical gradients required for hair cell function20. While there is strong evidence that this mechanism contributes to loss of cochlear integrity, we note that S1P2 is expressed in the hair cells and supporting cells of the cochlea, with expression increasing over time, coincident with the progression of the cochlear degeneration19. Therefore, we examined the possibility that additional, cell-intrinsic functions of S1P2 promote viability of cochlear structures.
The recent approval of fingolimod, a non-selective functional antagonist of four S1P receptors, for the treatment of multiple sclerosis23 demonstrates the feasibility of developing small molecule drugs that target S1P receptor signalling. Considering the pleotropic functions of the S1P receptor family, it would be a significant advantage to develop subtype-selective ligands as drug candidates. In this study, we demonstrate that activation of S1P2 is associated with reduction of ROS accumulation by a specific S1P2 agonist and provide proof-of-concept for its use as an otoprotective agent.
Results
Loss of S1P2 results in ROS generation and cochlear degeneration
We previously reported that S1pr2−/− knockout mice exhibit progressive degeneration of the sensory structures of the cochlea and vestibular end organs19. This is particularly evident in the spiral ganglia that innervate the organ of Corti. At 2 weeks of age, the neurons of the spiral ganglia are intact and indistinguishable from those of wild-type mice (Fig. 1A,B), but as previously described19, by 8 weeks of age there is marked degeneration of the ganglia at the basal turn of the cochlea, characterized by pronounced neuronal loss (Fig. 1C,D). To determine whether accumulation of ROS may contribute to this process, we evaluated wild-type and knockout cochlea for ROS content at 3 weeks of age prior to the onset of frank degeneration. Cochlea derived from S1pr2−/− mice were characterized by the formation of dense blue labeling in the area of the spiral ganglia following incubation with NBT, indicating accumulation of ROS in the afferent nerve fibers. By contrast, specific labeling of the spiral ganglia was absent in S1pr2+/− littermates (Fig. 1E–H).
S1P2 attenuates ROS accumulation in the cochlea.
Hematoxylin and eosin staining of cochlear tissue sections demonstrates structurally intact neurons in the spiral ganglia of S1pr2+/− (A) and S1pr2−/− (B) mice at 2 weeks of age. By 8 weeks of age, S1pr2+/− cochlea remain intact (C), but spiral ganglia of S1pr2−/− mice (D) demonstrate marked, progressive degeneration. (Results previously described19). The NBT assay demonstrates little staining in S1pr2+/− cochlea (E), but a consistent banding pattern in S1pr2−/− littermate cochlea (F), indicative of ROS accumulation. (G,H) Higher magnification reveals that the most intense staining is localized to the spiral ganglia. Images are representative of 7–9 mice per genotype. (I) In vitro assay for recombinant NOX3 activity reveals that S1P2, but not S1P1 or S1P3, can inhibit NOX3 activity in a ligand dependent manner. Co-transfection with constitutively active Rho, a known downstream mediator of S1P2 signalling, demonstrates a similar, but ligand-independent, inhibitory effect on NOX3. Scale bars represent 50 μm (A–D), 2 mm (E,F) and 1 mm (G,H), respectively. (*p < 0.05, **p < 0.01, ***p < 0.001).
To explain how loss of S1pr2 may lead to ROS accumulation, we sought to determine whether S1P2 could regulate the activity of NOX324,25. Transfection of HEK293 cells with the NOX3 complex resulted in a marked increase in ROS generation (Fig. 1I). This could be significantly attenuated by co-transfection with S1P2 and further attenuated by activation of S1P2 with S1P (1 μM). S1P-dependent reduction of ROS was not observed upon co-transfection with either of two other S1P receptor subtypes, S1P1 and S1P3. NOX3 activity was also similarly inhibited by co-transfection with constitutively-active RhoA (RhoA-CA), which is known to act downstream of S1P226. The use of RhoA-CA provides a useful control condition in that it 1) confirms that S1P2-regulated pathways inhibit NOX3 and 2) demonstrates the maximum inhibitory response that would be expected from activation of the RhoA pathway.
CYM-5478 is a potent, selective agonist for S1P2
To determine whether activation of S1P2 may be cytoprotective, we sought an S1P2-selective agonist. CYM-5478 was identified as a candidate S1P2 agonist in a high throughput screen by The Scripps Research Institute Molecular Screening Center (http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=872). This compound (PubChem CID: 7802604) had a reported EC50 of 723 nM in the original screen and an EC50 of 780 nM in subsequent validation studies27. We performed several cell-based assays to confirm this result and to evaluate the selectivity of CYM-5478. Use of a TGFα-shedding assay28 demonstrated that CYM-5478 activates S1P2 with an EC50 of 119 nM, but had less than 25% efficacy and showed 10-fold lower potency against the other S1P receptor subtypes (Fig. 2A–F). Control cells transfected with empty vector did not exhibit a measurable response when stimulated with either S1P or CYM-5478 (data not shown).
CYM-5478 is an S1P2-selective agonist.
The TGFα-shedding assay was performed to evaluate the activation of S1P1 (A), S1P2 (B), S1P3 (C), S1P4 (D) and S1P5 (E) by CYM-5478 relative to that of endogenous ligand, S1P. (F) A summary of the TGFα-shedding results demonstrates that only S1P2 is potently and effectively activated by CYM-5478. (G) MDA-MB-231 breast cancer cells typically display an elongated, fibroblast-like morphology (arrows), with few rounded cells (arrowheads), as visualized by labelling with fluorescein-phalloidin. (H) Upon stimulation with 1 μM S1P MDA-MB-231 cells become rounded. (I) Quantitation of cell rounding demonstrates that the response to CYM-5478 is equivalent to that of S1P and that the activity of both ligands can be inhibited by pre-treatment with S1P2 antagonist, JTE-013. (N = 3). S1P and CYM-5478 were evaluated for their ability to induce the internalization of S1P1-EGFP (J–L), S1P2-EGFP (M–O) and S1P3-EGFP (P–R) fusion proteins. (J,M,P) All receptors were predominantly localized to the plasma membrane (arrows) when treated with vehicle alone. (K,N,Q) All receptors were largely internalized to cytoplasmic vesicles (arrowheads) when stimulated with 1 μM S1P. (L,O,R) CYM-5478-treatment induced the internalization of S1P2-EGFP, but not S1P1-EGFP or S1P3-EGFP. Scale bars represent 100 μm (G,H) and 100 μm (J–R), respectively. (Images are representative of 3 independent experiments). (***p < 0.001).
To confirm that the activation of S1P2 was not an artefact of receptor overexpression, we evaluated the ability of CYM-5478 to activate endogenously expressed S1P2 receptors in MDA-MB-231 breast cancer cells. Upon stimulation with S1P (1 μM), serum-starved MDA-MB-231 cells displayed pronounced cytoskeletal rearrangement characterized by process retraction and cell rounding (Fig. 2G,H). This response was completely abrogated by pre-treatment with S1P2-selective antagonist JTE-01329. CYM-5478 elicited an identical rounding response in MDA-MB-231 cells, which was similarly abrogated by JTE-013 (Fig. 2I).
Activation of S1P receptor-EGFP constructs results in their internalization into cytoplasmic vesicles that can be visualized by fluorescence microscopy18. We exploited this effect to further validate the use of CYM-5478 as an S1P2-specific agonist. Both S1P (1 μM) and CYM-5478 (1 μM) were able to induce the translocation of S1P2 from the plasma membrane to cytoplasmic vesicles, but only S1P was effective against S1P1 and S1P3 (Fig. 2J–R).
CYM-5478 promotes viability and inhibits cell death in neural cells in vitro
Since loss of S1P2 results in progressive degeneration of sensory epithelial hair cells, supporting cells and afferent neurons of the cochlea19, we sought to determine whether activation of S1P2 could promote viability of a neural-derived cell line. C6 cells rat glioma cells30 were evaluated by RT-PCR and shown to express S1P2 as its predominant S1P receptor subtype (Fig. 3A). Under nutrient-deprivation stress produced by serum-starvation, CYM-5478 induced a statistically significant increase in the viability of C6 cells in a dose dependent manner at concentrations above 100 nM (Fig. 3B). This effect was absent in the presence of 10% fetal bovine serum (data not shown) suggesting that the increase in viability was a result of decreased starvation-induced cell death, rather than an increase in proliferation.
CYM-5478 promotes cell viability.
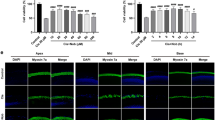
(A) qRT-PCR demonstrates that S1P2 is the predominant S1P receptor expressed in C6 cells. (B) When treated with CYM-5478, C6 cells demonstrated a dose-dependent increase in viability, as evaluated by MTT assay. (N = 6. Asterisks indicate significant differences compared to 0 μM CYM-5478). (C) C6 cells exhibit a dose-dependent decrease in viability when treated with cisplatin. Co-administration of 10μM CYM-5478 reduces sensitivity to cisplatin and confers a 3-fold increase in EC50. (D) C6 cells exhibit a >3-fold increase in propidium iodide-positive cells when treated with cisplatin. This effect is attenuated by co-administration of CYM-5478. There is no significant change in propidium iodide-positive cells due to CYM-5478-treatment alone. (N = 8). (E) Cisplatin induces a significant increase in caspase 3/7 activity in C6 cells. This effect is markedly attenuated by co-administration of CYM-5478. (N = 3). (**p < 0.01, ***p < 0.001)).
Since cisplatin is a known ototoxic compound that exerts its action, at least in part, by increased NOX3 activity3, we sought to determine whether activation of S1P2 could protect cells from cisplatin-mediated death. In the presence of CYM-5478 (10 μM) there was a statistically significant, 3-fold increase in the EC50 of cisplatin-mediated reduction in the viability of C6 glioma cells (Fig. 3C), consistent with pronounced cytoprotection produced by S1P2 activation. Similarly, CYM-5478 treatment was able to abrogate cisplatin-induced cell death, as evaluated by propidium iodide dye exclusion assay (Fig. 3D). We further found that this effect was coincident with the reduction of apoptosis. Treatment with CYM-5478 also attenuated cisplatin-induced caspase 3/7 activity in C6 cells (Fig. 3E). Taken together, these data demonstrate a significant cytoprotective effect through the activation of S1P2 by CYM-5478.
S1P2 activation inhibits the generation of ROS
To determine whether the cytoprotective effect of CYM-5478 was the result of decreased ROS production, we evaluated the sensitivity of C6 cells to cisplatin in the presence of a potent antioxidant, N-acetylcysteine31. When 1 mM N-acetylcysteine was added to culture media, there was no significant difference in the EC50 of cisplatin toward vehicle-treated or CYM-5478-treated cells (Fig. 4A), thus implicating ROS in the previously observed effect (Fig. 3C). To confirm that CYM-5478 is inhibiting the production of endogenous ROS and is not directly acting as an antioxidant, we evaluated the ability of CYM-5478 to protect C6 cells from exogenously administered ROS. C6 cells were equally sensitive to hydrogen peroxide in the presence and absence of CYM-5478 (Fig. 4B), indicating that CYM-5478 does not have significant antioxidant activity, consistent with its actions via S1P2. Using CellROX® reagent, we confirm that there is an increase in ROS in C6 cells that are exposed to cisplatin (20 μM) for 24 hours (Fig. 4C) and that this increase can be significantly attenuated by co-administration of CYM-5478 (10 μM) (Fig. 4D). Treatment of C6 cells with either JTE-013 (S1P2 antagonist) (Fig. 4E) or Y-27632 (Rho-associated protein kinase (ROCK) inhibitor) (Fig. 4F) had no effect on cisplatin-induced ROS, but resulted in a complete attenuation of CYM-5478 activity. This demonstrates that the protective effect of CYM-5478 is mediated by S1P2 and activation of Rho signaling. Furthermore, we show that the effect of CYM-5478 can be phenocopied by treatment with either NSC23766 (Rac1 inhibitor) (Fig. 4G) or diphenyleneiodonium (DPI, NADPH oxidase inhibitor) (Fig. 4H). This implicates Rac1 and NOX as likely targets that are inhibited by CYM-5478.
CYM-5478 acts via reduction of endogenous ROS.
(A) In the presence of 1 mM N-acetylcysteine, there is no significant difference in the sensitivity of CYM-5478-treated C6 cells compared to vehicle-treatment alone. (N = 6). (B) There is no significant difference in the sensitivity of C6 cells to hydrogen peroxide in the presence of CYM-5478 compared to vehicle-treatment alone. (N = 6). (C) Cisplatin-treatment causes increased fluorescence intensity of C6 cells treated with CellROX® reagent, indicative of ROS accumulation. (D) Quantification of CellROX® fluorescence demonstrates that co-administration of CYM-5478 (10 μM) attenuates the cisplatin-mediated ROS accumulation. (E–H) C6 cells were treated with cisplatin in the presence of small molecule inhibitors. Under all conditions, cisplatin induced a significant increase in ROS relative to vehicle-treated cells (p < 0.001). The protective effect of CYM-5478 is abolished by co-administration of either (E) S1P2 agonist, JTE-013, or (F) ROCK inhibitor, Y-27632. The CYM-5478-mediated attenuation of ROS accumulation can be phenocopied by treatment of C6 cells with either (G) Rac1 inhibitor, NSC23766, or (H) NADPH oxidase inhibitor, DPI. (N = 3) (I) Schematic representation of the likely signaling pathway by which S1P2 attenuates ROS accumulation. (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
The present study sought to identify cell-intrinsic phenomena that contribute to the cochlear degeneration that occurs in S1pr2−/− mice. We found that loss of S1P2 results in the accumulation of ROS. The Nox family of NADPH oxidases are unusual in that they produce ROS as their primary function rather than as a byproduct32 and are thus a major source of signalling ROS. It is not surprising, then, that the activity of these enzymes requires strict control by a regulatory complex, with subunits that include known second-messengers such as Rac1. This provides a mechanism by which receptor-mediated signal transduction can limit ROS generation. Our current study demonstrates for the first time that S1P2 activity inhibits NOX3, resulting in the reduction of cytotoxic ROS accumulation. It is likely that this occurs via activation of Rho26 and subsequent inhibition of Rac33, which is an obligate member of the NOX complex2.
Interestingly, several previous studies have found that S1P signalling can regulate NOX activity, but possible mechanisms are poorly characterized and often paradoxical. For example, while S1P signalling has been shown to increase ROS in fibroblasts9, vascular endothelial cells34 and isolated arteries8, S1P signalling has been shown to decrease ROS accumulation in vascular smooth muscle cells35. This apparent inconsistency in the literature is likely due to the heterogeneity of S1P signaling, much of which stems from differential receptor expression coupled with the fact that different S1P receptor subtypes activate different downstream signalling pathways. It is particularly notable that S1P1 exclusively activates Gαi26, which is a known activator of Rac1, whereas S1P2 is a strong inducer of Gα12/13, which activates Rho26. Therefore, S1P1 would be expected to activate NOX, whereas S1P2 should inhibit it (Fig. 4I). Furthermore, it was recently demonstrated that S1P complexed with high-density lipoprotein (ApoM) acts as a biased agonist, inducing distinctly different S1P1-mediated effects compared to albumin-bound S1P36. This implies that ligand presentation can affect S1P signaling and identifies an additional variable that may contribute to S1P otoprotective effects.
To underscore the translational relevance of this study, we have validated the activity of a recently identified S1P2 agonist, CYM-5478. This compound (PubChem SID #46371153, CID #7802604) was identified in a high throughput screen for S1P2 agonism and was used for SAR studies27, but was not systematically evaluated for receptor selectivity. In the current study, we provide the first demonstration that CYM-5478 is a potent and highly selective agonist for S1P2. In addition, we have used CYM-5478 to demonstrate that S1P2 mediates pro-migratory responses in oral squamous cell carcinoma (Patmanathan, manuscript under review), further demonstrating that CYM-5478 is a valuable tool for identifying biological functions of S1P2.
Interestingly, a recent study reports the identification of an autosomal-recessive nonsyndromic hearing impairment (ARNSHI) locus that contains the S1P2 gene (S1PR2)37. The authors further identify two mis-sense mutations that co-segregate with profound hearing loss in consanguineous families. This provides compelling evidence that the S1pr2−/− mouse is a faithful model for the role of S1P signalling in hearing loss.
These combined results suggest that S1P2 represents a potential drug target for the prevention of the ototoxicity caused by cisplatin. Despite the 75–100% occurrence of hearing loss associated with cisplatin therapy38, platinum-based chemotherapeutics remain first-line treatments for lung cancer and other tumor types. It is well-established that toxic accumulation of ROS is at least partly responsible for the effect of cisplatin on hearing loss39. Interestingly, the use of antioxidant therapy has shown some otoprotective potential, but has had limited success in actual clinical studies40. There are a number of issues that may be complicating this approach. For example, antioxidant therapy is non-selective and typically has a protective effect on tumor cells, thus interfering with the desired effect of cisplatin treatment. This can be partially ameliorated by careful adjustment of the dosing schedule39, or by transtympanic administration of the antioxidant41. As a targeted potential therapy, administration of an S1P2 agonist would be predicted to have increased selectivity for otoprotection, without known mechanisms for increasing tumor resistance to cisplatin. Furthermore, since S1P2 agonists would initially act extracellularly via cell-surface receptors to minimize endogenous ROS production, they should have higher potency and more favorable pharmacokinetics than antioxidants that must enter the cell and act on ROS that have already accumulated.
Further validation of the value of S1P2 as a therapeutic target was recently provided using an ex vivo culture model of gentamycin ototoxicity42. The authors demonstrated that administration of an antagonist for S1P2, but not S1P1 and S1P3, results in increased gentamycin ototoxicity43. They further show that inhibition of sphingosine kinase potentiates cisplatin ototoxicity and itself promotes apoptosis and hair cell loss44, which confirms the importance of endogenous S1P signalling in cochlear integrity. Additionally, S1P signalling has been implicated in other cellular and physiological functions in the inner ear45. Notably, S1P signalling has been shown to regulate cochlear blood flow and affect the integrity of the stria vascularis20,46. Future in vivo studies are required to further characterize S1P2 as a bona fide drug target for otoprotective therapy.
Methods
Materials
CYM-5478 (catalog #EN300-57094) was obtained from Enamine LLC. D-erythro-S1P (catalog #73914), N-acetylcysteine (catalog #A7250), hydrogen peroxide (catalog #216763), DPI (catalog #D2926), JTE-013 (catalog #J4080) and cisplatin (cis-Diammineplatinum(II) dichloride, catalog #P4394) were obtained from Sigma-Aldrich. Fluorescein phalloidin (catalog #F432), propidium iodide (catalog #P3566) and Hoechst 33342 (catalog #H3570) were obtained from Thermo Fisher Scientific. NSC23766 (item #13196) and Y-27632 (item #10005583) were obtained from Cayman Chemicals.
Animal Welfare and Ethical statement
Mice were housed in ventilated cages in the vivarium at The Scripps Research Institute (TSRI) on a 12 hour light/12 hour dark cycle, with ad libitum access to water and standard chow. S1pr2−/− mice were generated and maintained as described47 in a 129/SvJ, C57BL/6N mixed background. No procedures were performed on live animals for this study. All procedures were in compliance with state and federal regulations regarding animal welfare, followed the ARRIVE guidelines of the National Centre for the Replacement Refinement and Reduction of Animals in Research and were performed as humanely as possible. All procedures were approved the Institutional Animal Care and Use Committee at TSRI and complied with the US National Research Council’s “Guide for the Care and Use of Laboratory Animals,” and the US Public Health Service’s “Policy on Humane Care and Use of Laboratory Animals” and “Guide for the Care and Use of Laboratory Animals”.
Nitro blue tetrazolium (NBT) assay
Accumulation of ROS ex vivo was determined using a modification of a NBT assay previously described for visualization of ROS in cultured cells in situ48, in which the presence of ·O2− is detected by the conversion of NBT to a blue, insoluble formazan precipitate49. Cochlea were rapidly isolated and exposed by perforating the boney wall, washed in HBSS, incubated with NBT (1.6 mg/ml) in HBSS at 37 °C for precisely 45 min, then fixed in 100% methanol and photographed with a stereo microscope equipped with a Nikon Coolpix 950 camera.
Cell culture
C6 rat glioma cells (ATCC #CCL-107), MDA-MB-231 cells (ATCC #HTB-26) and HEK293 cells (ATCC #CRL-1573) were maintained as a monolayer culture on tissue culture dishes at 37 °C, 5% CO2, 100% humidity in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. S1P and CYM-5478 were solubilized with bovine serum albumin (0.1% final concentration) prior to treatment.
NOX3 activity assay
NOX3 activity was determined as previously described24,25. Briefly, HEK293 cells were transfected with expression constructs for the NOX3 complex (Nox3, NoxO1, NoxA1) and co-transfected with the indicated S1P receptor, incubated for 16 hours, then replated in 96-well plates in Hank’s Balanced Saline Solution. Cells were treated with S1P (1 μM) or vehicle and immediately evaluated for ROS by luminol assay. Indicated values were taken 15 minutes following stimulation.
Receptor internalization assay
Cells were cultured on collagen-coated coverslips (cat #08-115 Millipore), transfected with S1P receptor-enhanced green fluorescence protein (EGFP) fusion constructs using Lipofectamine 3000 reagent (cat #L3000001, Thermo Fisher) and serum-starved for 4 hours prior to ligand stimulation essentially as described50.
TGFα-shedding assay
The TGFα-shedding assay was performed essentially as described28. Briefly, HEK293 cells were co-transfected with the indicated receptor expression construct and TGFα-alkaline phosphatase using Lipofectamine 2000 reagent (cat #11668019, Thermo Fisher), collected by trypsinization, washed with phosphate-buffered saline and seeded into 96-well plates in Hank’s Balanced Saline Solution (HBSS). To improve assay sensitivity, S1P1 and S1P2 cells were co-transfected with Gαq/i1 chimeric protein and S1P4 and S1P5 were co-transfected with Gαq/16, as previously described28. Cells were stimulated with ligand for 1 hour, then alkaline phosphatase activity was detected in cells and in the supernatant. Receptor activity (% shedding) was defined as alkaline phosphatase activity of the supernatant/total alkaline phosphatase activity (cells + supernatant). Data processing and statistical analyses were performed with GraphPad Prism 6.
Cell rounding assay
Cell rounding was performed essentially as described51. MDA-MB-231 breast cancer cells were seeded on collagen-coated coverslips at 30–50% confluence, incubated overnight and serum-starved for 4 hours. They were then pretreated with JTE-013 or vehicle for 15 minutes and treated with S1P, CYM-5478, or vehicle. After 15 min, the cells were fixed and stained with fluorescein phalloidin and DAPI for cell morphology. The number of cells with retracted neurites and the number of total cells were counted in three separate fields for each sample and the percentage of cells with retracted neurites was calculated.
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from C6 glioma cells using Trizol reagent (Life Technologies) per manufacturer’s instructions. Approximately 2 μg of each sample was primed with oligo-dT and random hexamer primers prior using Thermo Scientific Maxima First Strand cDNA synthesis kit (Life Technologies). For quantitative real-time RT-PCR, targets were amplified with Maxima SYBR Green/ROX qPCR Master Mix (Life Technologies) on an Applied Biosystems ViiA 7 Real-Time PCR System (Life Technologies) using gene-specific primer pairs (Table 1). Quantitation was determined with a standard curve analysis as described52.
Viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described13. Briefly, C6 cells were seeded into 96-well plates at 20,000 cells/well, incubated overnight, serum-starved for 4 h and treated for 72 hours with cisplatin in the presence of CYM-5478 or vehicle.
Cell death assay
Cell death was evaluated by propidium iodide exclusion assay. Cells were plated in 96-well plates at ~50% confluence, incubated overnight, then treated with cisplatin (67 μM) for 24 hours in the presence of vehicle or 10 μM CYM-5478. Cell were treated with Hoechst 33342 (1 mg/ml) and propidium iodide (0.3 mg/ml) for 20 minutes, washed with phosphate-buffered saline, then rapidly photographed with a Cytation 3 automated imager (Biotek Instruments, Inc.). % cell death reflects a ratio of propidium iodide-positive and Hoechst 33342-positive cells.
Caspase assay
Cells were plated in clear-bottom, black-walled 96-well plates at ~50% confluence, incubated overnight, then treated overnight with cisplatin in the presence of vehicle or 10 μM CYM-5478. Caspase activation was evaluated by Caspase-Glo 3/7 Assay System (Promega Corporation) per manufacturer’s instructions.
ROS assay
Cells were plated in clear-bottom, black-walled 96-well plates at ~50% confluence, incubated overnight, then treated overnight with cisplatin in the presence of the indicated compounds. All conditions were vehicle-controlled (0.1% DMSO). Cells were treated with CellROX® Orange reagent (ThermoFisher #C10443) per manufacturer’s protocol and counter stained with Hoechst 33342 (ThermoFisher #62249). Cells were photographed with a 20X objective lens, 3 fields per well, 3 wells per condition. Quantification was performed using ImageJ software, but dividing the total integrated density of CellROX® labelling by the number of cells per Hoechst staining.
Additional Information
How to cite this article: Herr, D. R. et al. Sphingosine 1-phosphate receptor 2 (S1P2) attenuates reactive oxygen species formation and inhibits cell death: implications for otoprotective therapy. Sci. Rep. 6, 24541; doi: 10.1038/srep24541 (2016).
References
Oishi, N. & Schacht, J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs 16, 235–245, doi: 10.1517/14728214.2011.552427 (2011).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313, doi: 10.1152/physrev.00044.2005 (2007).
Banfi, B. et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 279, 46065–46072 (2004).
Kihara, Y., Maceyka, M., Spiegel, S. & Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 171, 3575–3594, doi: 10.1111/bph.12678 (2014).
Pyne, N. J. & Pyne, S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10, 489–503, doi: 10.1038/nrc2875 (2010).
Choi, J. W. & Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 1831, 20–32, doi: 10.1016/j.bbalip.2012.07.015 (2013).
Takuwa, N. et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc. Res. 85, 484–493, doi: 10.1093/cvr/cvp312 (2010).
Keller, M. et al. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J. 20, 702–704, doi: 10.1096/fj.05-4075fje (2006).
Catarzi, S. et al. Sphingosine 1-phosphate stimulation of NADPH oxidase activity: relationship with platelet-derived growth factor receptor and c-Src kinase. Biochim. Biophys. Acta 1770, 872–883, doi: 10.1016/j.bbagen.2007.01.008 (2007).
Golan, K. et al. S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood 119, 2478–2488, doi: 10.1182/blood-2011-06-358614 (2012).
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Herr, D. R. et al. Sphingosine 1-phosphate receptors are essential mediators of eyelid closure during embryonic development. J. Biol. Chem. 288, 29882–29889, doi: 10.1074/jbc.M113.510099 (2013).
Green, J. A. et al. The sphingosine 1-phosphate receptor S1P(2) maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat Immunol 12, 672–680, doi: 10.1038/ni.2047 (2011).
Jenne, C. N. et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 206, 2469–2481, doi: 10.1084/jem.20090525 (2009).
Chun, J. & Hartung, H. P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. neuropharmacol. 33, 91–101, doi: 10.1097/WNF.0b013e3181cbf825 (2010).
Chun, J. & Brinkmann, V. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov. med. 12, 213–228 (2011).
Choi, J. W. et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl. Acad. Sci. USA 108, 751–756, doi: 10.1073/pnas.1014154108 (2011).
Herr, D. R. et al. Sphingosine 1-phosphate (S1P) signaling is required for maintenance of hair cells mainly via activation of S1P2. J. Neurosci. 27, 1474–1478, doi: 10.1523/JNEUROSCI.4245-06.2007 (2007).
Kono, M. et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 282, 10690–10696 (2007).
MacLennan, A. J. et al. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res 220, 38–48, doi: 10.1016/j.heares.2006.06.016 (2006).
Chen, J. et al. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS genetics 10, e1004688, doi: 10.1371/journal.pgen.1004688 (2014).
Mutoh, T., Rivera, R. & Chun, J. Insights into the pharmacological relevance of lysophospholipid receptors. Br. J. Pharmacol. 165, 829–844, doi: 10.1111/j.1476-5381.2011.01622.x (2012).
Kim, J. S., Diebold, B. A., Babior, B. M., Knaus, U. G. & Bokoch, G. M. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J. Biol. Chem. 282, 34787–34800, doi: 10.1074/jbc.M704754200 (2007).
Kim, J. S. & Bokoch, G. M. Anthrax edema toxin inhibits Nox1-mediated formation of reactive oxygen species by colon epithelial cells. J Innate Immun 1, 145–152, doi: 10.1159/000151481 (2009).
Windh, R. T. et al. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3 and H218/Edg-5 to the G(i), G(q) and G(12) families of heterotrimeric G proteins. J. Biol. Chem. 274, 27351–27358 (1999).
Satsu, H. et al. A sphingosine 1-phosphate receptor 2 selective allosteric agonist. Bioorg. med. chem. 21, 5373–5382, doi: 10.1016/j.bmc.2013.06.012 (2013).
Inoue, A. et al. TGFalpha shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. methods 9, 1021–1029, doi: 10.1038/nmeth.2172 (2012).
Osada, M., Yatomi, Y., Ohmori, T., Ikeda, H. & Ozaki, Y. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem. Biophys. Res. Commun. 299, 483–487 (2002).
Benda, P., Lightbody, J., Sato, G., Levine, L. & Sweet, W. Differentiated rat glial cell strain in tissue culture. Science 161, 370–371 (1968).
Aruoma, O. I., Halliwell, B., Hoey, B. M. & Butler, J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free radic. biol. med. 6, 593–597 (1989).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews 87, 245–313, doi: 10.1152/physrev.00044.2005 (2007).
Burridge, K. & Wennerberg, K. Rho and Rac take center stage. Cell 116, 167–179 (2004).
Harijith, A. et al. Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins. Am. J. Pathol. 183, 1169–1182, doi: 10.1016/j.ajpath.2013.06.018 (2013).
Tolle, M. et al. HDL-associated lysosphingolipids inhibit NAD(P)H oxidase-dependent monocyte chemoattractant protein-1 production. Arterioscler Thromb Vasc Biol 28, 1542–1548, doi: 10.1161/ATVBAHA.107.161042 (2008).
Galvani, S. et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. signal. 8, ra79, doi: 10.1126/scisignal.aaa2581 (2015).
Santos-Cortez, R. L. et al. Autosomal-Recessive Hearing Impairment due to Rare Missense Variants within S1PR2. Am. J. Hum. Genet., doi: 10.1016/j.ajhg.2015.12.004 (2016).
McKeage, M. J. Comparative adverse effect profiles of platinum drugs. Drug saf. 13, 228–244 (1995).
Rybak, L. P., Whitworth, C. A., Mukherjea, D. & Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res 226, 157–167 (2007).
Brock, P. R. et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. clin. oncol. 30, 2408–2417, doi: 10.1200/JCO.2011.39.1110 (2012).
Riga, M. G. et al. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity: a feasible method with promising efficacy. Am. j. clin. oncol. 36, 1–6, doi: 10.1097/COC.0b013e31822e006d (2013).
Nishimura, B., Tabuchi, K., Nakamagoe, M. & Hara, A. The influences of sphingolipid metabolites on gentamicin-induced hair cell loss of the rat cochlea. Neurosci. Lett. 485, 1–5, doi: 10.1016/j.neulet.2010.08.014 (2010).
Nakayama, M. et al. The influence of sphingosine-1-phosphate receptor antagonists on gentamicin-induced hair cell loss of the rat cochlea. Neurosci. Lett. 561, 91–95, doi: 10.1016/j.neulet.2013.12.063 (2014).
Tani, K., Tabuchi, K. & Hara, A. Hair Cell Loss Induced by Sphingosine and a Sphingosine Kinase Inhibitor in the Rat Cochlea. Neurotox. res., doi: 10.1007/s12640-015-9563-7 (2015).
Romero-Guevara, R., Cencetti, F., Donati, C. & Bruni, P. Sphingosine 1-phosphate signaling pathway in inner ear biology. New therapeutic strategies for hearing loss? Front. aging neurosci. 7, 60, doi: 10.3389/fnagi.2015.00060 (2015).
Scherer, E. Q. et al. Sphingosine-1-phosphate modulates spiral modiolar artery tone: A potential role in vascular-based inner ear pathologies? Cardiovasc. Res. 70, 79–87 (2006).
Ishii, I. et al. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J. Biol. Chem. 277, 25152–25159. Epub 22002 May 25152. (2002).
Serrander, L. et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 406, 105–114, doi: 10.1042/BJ20061903 (2007).
Bartosz, G. Use of spectroscopic probes for detection of reactive oxygen species. Clin. Chim. Acta 368, 53–76, doi: 10.1016/j.cca.2005.12.039 (2006).
Harris, G. L., Creason, M. B., Brulte, G. B. & Herr, D. R. In Vitro and In Vivo Antagonism of a G Protein-Coupled Receptor (S1P3) with a Novel Blocking Monoclonal Antibody. PLoS One 7, e35129, doi: 10.1371/journal.pone.0035129 (2012).
Herr, K. J., Herr, D. R., Lee, C. W., Noguchi, K. & Chun, J. Stereotyped fetal brain disorganization is induced by hypoxia and requires lysophosphatidic acid receptor 1 (LPA1) signaling. Proc. Natl. Acad. Sci. USA 108, 15444–15449, doi: 10.1073/pnas.1106129108 (2011).
Dubin, A. E., Herr, D. R. & Chun, J. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J. Neurosci. 30, 7300–7309, doi: 10.1523/JNEUROSCI.6151-09.2010 (2010).
Acknowledgements
The authors would like to thank Gary Bokoch for technical assistance with NOX3 activity assays. This work was supported by National Institutes of Health grant DA019674 (JC), the Capita Foundation (DRH), the Ministry of Education, Singapore (DRH), the National University of Singapore (DRH) and University of Malaya-MOHE High Impact Research Grant UM.C/625/1/HIR/MOHE/DENT/10 (ICP).
Author information
Authors and Affiliations
Contributions
D.R.H., M.J.Y.R., Y.X.P., W.W., J.S.K., C.W.L. and R.R. performed the research. D.R.H., I.C.P. and J.C. designed the research study. J.C. contributed essential reagents or tools and manuscript editing. D.R.H., I.C.P. and J.C. analysed the data. D.R.H. and I.C.P. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Herr, D., Reolo, M., Peh, Y. et al. Sphingosine 1-phosphate receptor 2 (S1P2) attenuates reactive oxygen species formation and inhibits cell death: implications for otoprotective therapy. Sci Rep 6, 24541 (2016). https://doi.org/10.1038/srep24541
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24541
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.