Abstract
Boreal forests comprise 73% of the world’s coniferous forests. Based on forest floor measurements, they have been considered a significant natural sink of methane (CH4) and a natural source of nitrous oxide (N2O), both of which are important greenhouse gases. However, the role of trees, especially conifers, in ecosystem N2O and CH4 exchange is only poorly understood. We show for the first time that mature Scots pine (Pinus sylvestris L.) trees consistently emit N2O and CH4 from both stems and shoots. The shoot fluxes of N2O and CH4 exceeded the stem flux rates by 16 and 41 times, respectively. Moreover, higher stem N2O and CH4 fluxes were observed from wet than from dry areas of the forest. The N2O release from boreal pine forests may thus be underestimated and the uptake of CH4 may be overestimated when ecosystem flux calculations are based solely on forest floor measurements. The contribution of pine trees to the N2O and CH4 exchange of the boreal pine forest seems to increase considerably under high soil water content, thus highlighting the urgent need to include tree-emissions in greenhouse gas emission inventories.
Similar content being viewed by others
Introduction
Methane (CH4) and nitrous oxide (N2O) are naturally produced in soils. The net CH4 and N2O flux at the soil–atmosphere interface is a balance of gas production, consumption and transport processes within soil (Supplementary Fig. S1). CH4 is produced by anaerobic methanogenesis1 in water saturated soils and oxidized by methanotrophic bacteria2. N2O is mainly formed during denitrification, anaerobic dissimilatory nitrate reduction to ammonium and aerobic nitrification1. Denitrification is the only process consuming N2O by reduction to N2.
In addition to gas diffusion at the soil surface and ebullition1, it has been shown that plant-mediated transport3,4,5,6,7,8,9,10,11,12,13 can contribute significantly to CH4 and N2O exchange between the pedosphere and the atmosphere (Supplementary Fig. S1). CH4 and N2O produced in the soil can be taken up by roots, diffuse across root cortex3,6 and be transported into the above-ground plant tissues. This transport occurs via intercellular spaces and the aerenchyma system3,5,6,7,11 and/or in xylem via the transpiration stream4,5,8,13. Release of CH4 and N2O into the atmosphere takes place via lenticels or stomata3,6,11,13. Both gases may also be formed in plants, either by microorganisms living within the plant14,15,16 or by physiological and photochemical processes17,18,19.
In recent decades, N2O and CH4 fluxes from plants have predominantly been investigated in herbaceous plants from wetlands. Studies in trees are rather rare and restricted mostly to stem flux measurements on wetland species. Particularly, those upland tree species lacking an aerenchyma system have been poorly investigated8,9,13,20. This is despite the fact that upland soils seem to be an important natural source of N2O21 and a strong natural sink of CH422. Moreover, the current flux estimates of N2O and CH4 from forest ecosystems are based mostly on measurements from the forest floor, excluding the contribution of trees.
We quantified N2O and CH4 fluxes from stems, shoots (i.e. terminal branches of ca 15–20 cm length in upper canopy) and the forest floor of boreal forest dominated by Scots pine (Pinus sylvestris L.). We also investigated whether soil moisture level affects the N2O and CH4 exchange from trees and forest floor. This study is unique for its simultaneous determination of stem, shoot and forest floor fluxes. Data were collected during May to July 2013 in a 50-year-old P. sylvestris stand23 in Southern Finland on two experimental plots (dimensions of 20 × 15 m, a distance of 100 m apart) with naturally differing soil volumetric water content (VWC): dry plot with 0.33 ± 0.030 m3 m−3, wet plot with 0.75 ± 0.016 m3 m−3 (mean ± standard error).
Results and Discussion
N2O fluxes
In dry field conditions typical for the studied boreal forest, we observed that P. sylvestris stems and shoots emitted N2O at rates (medians) of 0.023 and 0.097 μg N2O per m2 of stem and projected leaf area, respectively, per hour (Supplementary Fig. S2a), accounting for 0.11 and 1.9 mg N2O, respectively, after scaling up per hectare of ground area per hour (see Methods, Fig. 1a). To our knowledge, measurements of shoot fluxes of N2O from mature trees have never been reported and most studies assume negligible shoot emissions compared to stem fluxes5,9,10,12,13. Contrary to this current understanding, the shoot fluxes of N2O from the studied pine trees exceeded the stem fluxes by more than 16 times. This underlines the important role of forest canopies in trace gas exchange. The N2O fluxes from pine trees were accompanied by forest floor flux rates reaching 2.50 μg N2O m−2 h−1 (24.9 mg N2O ha−1 h−1; Supplementary Fig. S2a, Fig. 1a), which agrees with previous soil N2O measurements in the same forest24. In general, boreal forest soils are characterized by low availability of mineral N23,25 and low N deposition23, resulting in low soil N2O emissions, particularly when compared to 4 to 12 times higher emissions from temperate and tropical forests25.
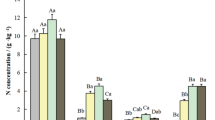
Scaled-up forest floor, stem and shoot fluxes of N2O (a) and CH4 (b) per unit ground area of dry boreal forest stand, dominated by Scots pine (Pinus sylvestris).
Original flux rates per surface area of each ecosystem part are presented in Supplementary Fig. S2. Solid lines within the boxes mark medians, broken lines denote means, boundaries indicate 25th and 75th percentiles and the whiskers 10th and 90th percentiles. Dots mark outliers. The plotted results are the medians/means of all sampling locations from the dry plot as follows: Forest floor fluxes are determined as medians and means of measurements from three soil chambers (n = 3) with nine measurement repetitions per chamber. Stem and shoot fluxes are expressed as medians and means of measurements on three trees (n = 3) with four to six repetitions per chamber. The fluxes from the shoots, stems and from the forest floor were measured simultaneously to allow their comparison. Contribution of stems and shoots to N2O and CH4 exchange are expressed as percentage of the forest floor flux. Statistically significant differences at p < 0.017 (multiple comparison – Bonferroni correction) between flux components are indicated by different capital letters above bars.
The up-scaled N2O emission rates from trees, assuming the mean tree constitution and density of 1000 trees per hectare in the dry plot (see Methods), were equivalent to 8.0% of the forest floor emissions per hectare of ground area (Fig. 1a, comparison of medians). Thus, the N2O emissions from trees constitute a significant part of the boreal pine forest N2O flux. The N2O flux from dry areas of the studied forest, including the contribution from forest floor and pine trees, reached approximately 26.9 mg N2O ha−1 h−1 (8.0 g CO2-e ha−1 h−1 using a global warming potential [GWP] of 298 [ref. 26]), which lies within the range of the global inventory estimates of N2O flux rates for boreal forests25. Based on the shoot-to-stem N2O fluxes ratio of 16 at the dry area, the shoot fluxes at the wet plot could reach 3.3 mg N2O ha−1 h−1 versus the measured stem fluxes of 0.20 mg N2O ha−1 h−1. As follows, under high soil water content typical for studied wet areas of the forest with density of 1400 trees per hectare, the contribution of pine trees could be up to 18% (based on medians comparison) of the forest floor N2O exchange.
Naturally, the up-scaled fluxes include uncertainties stemming from e.g. spatio-temporal variability in the fluxes, the use of mean stand density and constant shoot-to-stem flux ratio from the dry plot. The use of the constant shoot-to-stem flux ratio is justified based on the assumption that transport of N2O via the transpiration stream is the main driver for N2O emissions from the tree canopy8 and hence the stem emissions are directly reflected in the emissions from the canopy. Forest floor N2O and CH4 exchange is often characterized by high spatial variability, which has been also found to vary with distance to the trees27,28, while the variation in canopy N2O/CH4 exchange between individual pine trees as well as between different tree species remain unknown due to lack of canopy flux measurements. We estimated that temporal variability in the shoot, stem and forest floor N2O and CH4 fluxes was higher than the spatial variability in the dry plot, whereas in the wet plot the spatial variability dominated the fluxes. This indicates that most of the variability in the fluxes in the dominating dry areas originates from day-to-day variation, whereas the fluxes in the wet areas, which form a minority of the forest, are dominated by high small-scale variation.
The pine stem N2O fluxes correlated positively with forest floor fluxes (Spearman’s rank correlation coefficient: ρ = 0.351, p < 0.05), indicating that the tree-emitted N2O could originate from soil. As N2O is rather water soluble4 and many plant species emit N2O irrespective of the presence of an aerenchyma system4,5,7,8,9,13, we hypothesize that N2O is absorbed by roots from the soil, transported via xylem into the above-ground tree parts and then emitted into the atmosphere.
CH4 fluxes
Contrary to the CH4 uptake by shoots (i.e. negative flux) found in Scots pine seedlings grown under field and laboratory conditions20, we observed emissions of CH4 from both shoots and stems of mature P. sylvestris. This difference in shoot CH4 fluxes may result from (i) different soil water content and soil temperature (not reported for the seedlings experiment20), (ii) known discrepancy in emission capacities of young and mature trees12 and (iii) the fact that the seedlings were investigated in the absence of UV radiation20, which is known to stimulate CH4 formation17,18. The CH4 emission rates from pine stems and shoots were 0.005 and 0.050 μg CH4 m−2 h−1 (medians), respectively (Supplementary Fig. S2b). Up- scaled emission rates at stand level were 0.03 and 1.1 mg CH4 ha−1 h−1 (Fig. 1b) assuming mean tree constitution and density of 1000 trees per hectare (see Methods). As is the case of N2O, pine shoots seem to be the primary tree surface emitting CH4 into the atmosphere, given that shoot fluxes were 41 times higher than the stem fluxes. This contradicts the common assumption5,9,10,11 that basal regions of stems are the main source of CH4 and N2O from trees.
Whereas trees were a source of CH4, the forest floor was a sink (−14.4 μg CH4 m−2 h−1, Supplementary Fig. S2b; −143 mg CH4 ha−1 h−1, Fig. 1b). The estimated average pine tree CH4 emission represented 0.8% of the forest floor uptake. The CH4 uptake from the dry area of the studied forest (−4.9 g CO2-e ha−1 h−1 using GWP of 34 [ref. 26]) is roughly 35% to 50% lower than are estimates of CH4 uptake for boreal forests in global inventories25,29.
The median stem CH4 fluxes at the wet plot (0.100 μg CH4 m−2 h−1) were one order of magnitude higher than those at the dry plot (0.013 μg CH4 m−2 h−1) (Fig. 2b), while the soil remained a sink for CH4 even under high soil VWC (−7.09 μg CH4 m−2 h−1, −70.7 mg CH4 ha−1 h−1; Fig. 2d). Moreover, the stem-to-forest-floor CH4 fluxes ratio increased with soil VWC, underlining the importance of pine trees at wet areas in the balance of CH4. Although direct measurement of shoot CH4 flux at the wet plot was technically impossible, based on the shoot-to-stem CH4 fluxes ratio of 41 at the dry plot, the shoot CH4 fluxes at the wet plot were estimated to reach 24 mg CH4 ha−1 h−1 in comparison to the stem CH4 fluxes of 0.59 mg CH4 ha−1 h−1. Under high soil VWC and stand density of 1400 trees per hectare, CH4 emissions from pine trees could, therefore, account for up to 35% of the forest floor uptake. This estimate is rather higher than in a recent study by Pangala and colleagues, who found that CH4 emissions mediated by Alnus glutinosa and Betula pubescens contribute up to 14% to the total CH4 fluxes from a temperate forested wetland12.
Stem and forest floor fluxes of N2O (a,c) and CH4 (b,d) from dry and wet plots of boreal forest dominated by Scots pine (Pinus sylvestris).
Mean volumetric water contents for dry and wet plots (±s.e.) were 0.33 ± 0.030 m3 m−3 and 0.75 ± 0.016 m3 m−3, respectively. Flux rates of all sampling points as follows are expressed as medians (solid lines) or means (broken lines) per m2 of surface area. Stem fluxes are determined from six trees per plot (n = 6; 3–6 measurement repetitions per tree) and forest floor fluxes from three soil chambers per plot (n = 3; 6–9 repetitions per chamber). The fluxes from the stems and from the forest floor were always measured simultaneously. Statistically significant differences at p < 0.05 are indicated by an asterisk. For box plots description, see Fig. 1.
The partial soil origin of pine-emitted CH4 is supported by strong positive correlation of stem CH4 fluxes with forest floor CH4 fluxes (ρ = 0.716, p < 0.001) and VWC in topsoil (ρ = 0.802, p < 0.001). In wet conditions, pine trees may therefore prevent CH4 consumption in the upper soil layers by transporting CH4, produced in deeper soil, into the atmosphere. We suggest that soil-produced CH4 is transported into the above-ground parts of P. sylvestris mainly by the transpiration stream and then released into the atmosphere predominantly via stomata4,5,8,9, thus explaining the higher CH4 emissions from shoots as compared to stems. This assumption is supported by the positive correlation between the shoot CH4 flux and transpiration (ρ = 0.626, p < 0.05) and stem CH4 flux and sap flow (ρ = 0.390, p < 0.01). Therefore, alternative pathways, such as radial diffusion of CH4 (and N2O) in stems through intercellular spaces of the ray parenchyma and a release from the stem via lenticels11,30,31, seem of a lesser importance.
Different mechanisms of CH4 emissions from trees grown on dry plot as compared to those on wet plot are, however, likely. Limited soil CH4 production in deeper mineral soil layers32 and low mineral soil VWC (0.28 ± 0.02 m3 m−3) in the studied period give an assumption of negligible soil CH4 production in the dry plot. Moreover, approximately half of the root system of P. sylvestris is located in the top soil organic layer with the rest of the roots equally distributed to mineral soil (0–40 cm)33. Therefore, it is probable that part of the CH4 emitted from trees in the dry plot originated from anaerobic production processes within the wood14,15,16 and/or aerobic, non-microbial metabolic processes in the plant tissues17,18.
P. sylvestris appears to be one of the missing sources for N2O and CH4 in boreal forests. N2O emissions from boreal pine forests may previously have been underestimated and the uptake of CH4 overestimated. Even though our measurements indicate only potential mechanisms and more detailed measurements of spatio-temporal variability are necessary, the pine mediated N2O and CH4 emissions could account for up to 18% of forest floor N2O emissions and 35% of forest floor CH4 uptake, respectively, under high soil moisture conditions. This can be crucial for the future greenhouse gas budgets of boreal pine forests, especially if precipitation and evapotranspiration patterns will change due to climate change. Our findings highlight the important, but often neglected role of upland trees in N2O and CH4 exchange between the biosphere and the atmosphere and the importance of including tree emissions to the total forest ecosystem budgets of N2O and CH4.
Methods
Site description and experimental design
The measurements were performed in a 50-year-old stand of Scots pine (Pinus sylvestris L.) at the SMEAR II station (Station for Measuring Ecosystem–Atmosphere Relations) in Hyytiälä, Southern Finland (61°51′N, 24°17′E, 181 m a.s.l.) from 23 May to 19 July 2013. Established in 1962, the site is a boreal coniferous forest dominated by P. sylvestris with some additional Norway spruce (Picea abies) and broadleaved trees in the understorey23,34. The long-term annual mean temperature and precipitation are 3.5 °C and 711 mm, respectively35. The soil is Haplic podzol on glacial till with irregularly distributed peat soil spots36.
Naturally wet and dry plots (dimensions of 20 × 15 m, a distance of 100 m apart) with mean soil volumetric water content (VWC) 0.75 ± 0.016 m3 m−3 (mean ± standard error) and 0.33 ± 0.030 m3 m−3, respectively, were selected. During the measurement period, soil water content was measured using an HH2 Moisture Meter and Theta Probe (type ML2x, AT Delta-T Devices, Cambridge, UK) in A-horizon corresponding to depths 0–5 cm from the soil surface and expressed as mean of three independent measurements close to each tree and soil chamber. Soil temperature was measured continuously by a DS1921G Maxim Thermochron iButtons (Maxim Integrated, San Jose, California, USA) in A-horizon next to each soil chamber.
On each plot, six representative trees were chosen for stem flux measurements (n = 6). Shoot fluxes were measured from the upper canopy of three trees used for the stem flux measurements at the dry plot. Shoot fluxes were not measured from the wet plot as installation of a scaffold tower was technically not possible. Forest floor CH4 and N2O fluxes were measured at three representative positions in the dry plot and at three positions in the wet plot (n = 3).
Four flux measurement campaigns, each taken over ca 2 weeks, were made for stem, shoot and forest floor fluxes between 23 May and 19 July 2013 (for details concerning the number of replicates see the legends of Figs 1 and 2 and Supplementary Fig. S2 describing individual measuring campaigns). Simultaneous measurements of fluxes from each tree and the forest floor in its vicinity allowed a comparison of N2O and CH4 fluxes between tree shoots, stems and forest floor. The fluxes were determined on all measuring days at approximately same time to prevent possible variation in flux rates caused by flux diurnal cycle.
The mean tree height, length of living crown and stem diameter at breast height (DBH) of the selected pine trees were 18.2 ± 0.4 m, 6.47 ± 0.32 m and 0.162 ± 0.012 m, respectively, for the wet plot. For the dry plot, these were 17.7 ± 0.5 m, 7.22 ± 0.43 m and 0.180 ± 0.004 m, respectively. These morphological parameters did not differ significantly when comparing wet and dry plots. The stand densities were estimated to be 1000 and 1400 trees per hectare on the dry and wet plots, respectively. The stand basal area was measured directly on the plots using a rod relascope technique and was 19.5 and 26 m2 ha−1 on dry and wet plots, respectively.
Chamber systems
The stem fluxes were measured using 12 stem chambers (1 chamber per tree) enclosing the entire stem circumference37-modified. The skeleton of the stem chamber (volume between 0.0009 and 0.0015 m3 depending on stem diameter) was created by a flexible pipe from polyethylene-coated aluminium (Synflex, Eaton Hydraulics Group Europe, Morges, Switzerland), which was wrapped in a spiral around the stem. A tube-fitting brace was attached to this spiral and enabled fixation of inlet and outlet connectors. Teflon FEP film (0.05 mm thick, Fluorplast, Maalahti, Finland) impermeable for CH4 and N2O was wrapped 1.5 to 2 times around the tube spiral to create the chamber wall and then sealed with adhesive FEP tape. Due to the requirement of mounting the stem chambers on the basal part of the rough pine bark (around 0.2 m above the forest floor), the surface of the dead outer bark was carefully removed from the upper and basal ends of the chamber. The upper and basal ends of the Teflon foil were sealed with elastic closed cell polyethylene foam and wide flexible ties to the carefully smoothed bark surface. The results of the stem flux measurements on twelve trees were used in the comparison of the stem and forest floor fluxes between dry and wet plots (Fig. 2).
Two different shoot chamber types were used to measure fluxes of CH4 and N2O: two cylindrical chambers with FEP foil walls38 (volume 0.0054 m3) and a methacrylic cylindrical shoot chamber39 (volume 0.005 m3). We did not observe any differences in flux rates obtained by these two types of chambers. The three chambers were installed in the upper canopy of the three trees on the dry plot. The air temperatures (DT 612 thermometer, CEM, Shenzhen, China) inside and outside of the chambers were regularly measured during chamber closures. To avoid overheating in the chambers, the shoot fluxes were measured only on cloudy days. The comparison of the shoot, stem and forest floor fluxes presented in Fig. 1 and Supplementary Fig. S2 is based on the measurements at the dry plot only. To compare the whole tree flux rates in dry and wet plots, we used the shoot-to-stem flux ratio from the dry plot where both shoot and stem flux measurements were performed.
In both stem and shoot chambers, the mixing of the air inside the chambers was provided by vacuum pumps (V 1500-GAS-12V standard vacuum pumps, Xavitech, Härnösand, Sweden; NMP 850.1.2. KNDC B, KNF Neuberger, Freiburg, Germany) gas-tightly connected to the chamber using Teflon tubes and stainless steel connectors (Swagelok, Ohio, USA). The chambers were non-steady-state flow-through chambers returning the air from the pump again into the chambers. Gas samples were taken with a syringe via a septum connected to the air circulation. Six gas samples (each 20 ml) were taken from the closed stem and shoot chambers at time intervals of ca 60 min over a period of 6 h. The possible under-pressure resulted from the gas sampling was compensated by the flexible foil wall. The stem chambers were flushed with ambient air for at least 30 min before sampling.
Forest floor CH4 and N2O fluxes were measured using large opaque soil chambers made of aluminium40- chamber #13. Three chambers were placed on the dry plot (volume of ca 0.091 m3 depending on vegetation inside the chamber, enclosed soil surface area of 0.298 m2) and three on the wet plot (volume of ca 0.133 m3, soil area of 0.298 m2). The chambers were located in the vicinity of the measured trees. The ground vegetation in the soil chambers varied among chambers depending on the soil conditions and location and consisted of Sphagnum sp., Polytrichum sp., Dicranum polysetum, Pleurozium schreberi, Equisetum sylvaticum, Vaccinium myrtillus, Vaccinium vitis-idaea, Trientalis europaea and several representatives of Poaceae. The placement of chamber collars took place several days before the first sampling to allow the soil to settle and avoid soil disturbances. The soil chambers were closed for ca 40 min during which gas samples (each 20 ml) were taken at 2, 5, 10, 20, 30 and 40 minutes after the chamber closure. A fan was used to mix the headspace air during the closure. Chamber headspace temperature (DT 612 thermometer, CEM, China) was regularly monitored during the measurements.
Gas analyses
Gas samples from stem, shoot and soil chambers were taken in 20 ml syringes (BD syringe, Franklin Lakes, New Jersey, USA) and immediately transferred to the evacuated 12 ml glass vials (Labco, Ceredigion, UK), then stored at 4 °C. The gas samples were analysed by an Agilent 7890A gas chromatograph (GC) (Agilent Technologies, Santa Clara, California, USA) equipped with a flame ionization detector (FID) and an electron capture detector (ECD) for CH4 and N2O analyses, respectively40. Briefly, CH4 was detected by FID (300 °C) supplied with synthetic air (450 ml min−1) and hydrogen (H2, 45 ml min−1) and with nitrogen (N2, 5 ml min−1) as a make-up gas. N2O was detected using the ECD (380 °C) supplied with argon/methane (15 ml min−1) as a make-up gas. Helium (He, 45 ml min−1) was used in both cases as a carrier gas. Columns Porapak Q 80–100 Mesh and Hayesep Q 80–100 Mesh (Agilent Technologies, USA) were used for water vapour removal and gas separation. Oven temperature was kept at 60 °C. Retention times for CH4 and N2O were 3.6 and 4.3 min, respectively. The gas samples were automatically injected by an autosampler Gilson GX-271 Liquid Handler (Gilson, Middleton, Wisconsin, USA). An overpressure in vials was necessary for proper injection of gas samples and was an indicator of gas tightness of the vials. ChemStation B.03.02 software was used for the GC analyses.
The identification of CH4 and N2O peaks in gas samples and calculation of their molar fractions referred to dry air (hereinafter: “concentrations”) were performed using a four-point standard curve with the following concentrations: CH4 (1.207, 1.810, 2.413, 3.017 ppm of CH4 in synthetic air), N2O (0.279, 0.330, 0.381, 0.457 ppm of N2O in synthetic air). The four standards were analysed at the beginning of the analyses and after every ca 30 gas samples. A running standard (1.810 ppm CH4, 0.330 ppm N2O; in synthetic air) for detailed control was applied after every ca 15 gas samples.
Calculation of N2O and CH4 flux rates
The flux rates of N2O and CH4 from stems, shoots and forest floor were calculated by linear least square fits of time series of N2O and CH4 concentrations as follows

where F is flux of N2O or CH4 from stem, shoot, or forest floor surface [μg m−2 (surface area) h−1]; S is the slope of the linear fit to the N2O or CH4 concentrations over the chamber closure (ppm s−1); V is volume of chamber [m3]; A is stem surface area, projected leaf area, or soil surface area enclosed in stem, shoot, or soil chamber, respectively [m2]; M is molecular mass of N2O or CH4 [44.01 and 16.04 g mol−1, respectively]; Vm is molar volume of an ideal gas at 1 atmosphere pressure and 25 °C [0.0245 m3 mol−1]; and T the temperature [°C] inside the chamber. The stem surface area was estimated as a smooth cylinder around the bark because the micro-topography of the bark (very rough surface) makes any other methods ambiguous. The projected leaf area of shoots enclosed in the shoot chambers was determined by applying a destructive method at the end of the measurement campaign using an LI-3000 portable area meter (Li-Cor, Lincoln, Nebraska, USA).
The flux rates of N2O and CH4 were further estimated for the entire stem and projected needle area of each tree using the following parameters: The stem surface area (3.6–6.2 m2 per tree) was calculated as the lateral surface area of a right circular cone using the stem diameter at breast height (DBH) and the tree height. The needle biomass was determined using an allometric biomass equation (based on DBH, tree height and length of living crown) for Scots pine41-equation n. 27 and used to calculate the entire projected needle area of each tree (10–31 m2 per tree) by multiplying the biomass weight with specific leaf area determined for P. sylvestris at the SMEAR II station42. The CH4 and N2O fluxes from stems, shoots and forest floor were scaled up to 1 hectare of 50-year-old boreal pine stand using the estimated forest density and stand basal area (see chapter “Site description”).
The flux estimates and upscaling to a stand level are saddled with uncertainties arising from sampling and gas analyses43, variables in Equation 1 and application of allometric relationships for estimation of total leaf area41 and from calculation of stem area per tree. At a stand level, uncertainties of flux rates are thus particularly given by spatio-temporal variability in the fluxes, heterogeneity of tree morphological parameters (height, length of living crown, stem diameter) and stand heterogeneity (tree density per hectare and species composition etc.). In addition, fast changes in transpiration and sap flow rates induced by dynamic light environment under variable sky conditions have also a potential to substantially influence the gas exchange over longer periods44. However, here such factors are of minor importance, as the measurements were predominantly conducted during overcast days.
Ancillary measurements
The following continuously measured variables at the SMEAR II experimental station were used for correlation analyses: a) soil water content (TDR-100, Campbell Scientific, North Logan, Utah, USA)45,46 and b) soil temperature (Philips KTY81, NXP, Eindhoven, Netherlands)46, both in four soil horizons (O-, A-, B- and C-horizon; corresponding to depths of −4–0, 0–5, 5–23 and 23–60 cm from the mineral soil surface); c) air temperature at 4.2 m height within the forest stand (Pt100 sensors), d) photosynthetic photon flux density at 23 m height (Li-190SZ, Li-Cor, USA); on P. sylvestris: e) stem sap flow using the Granier-type heat dissipation method47,48 at a height of about 2 m; and f) shoot transpiration at the top canopy with dynamic enclosures49.
Statistics
Datasets were tested for normal distribution (Shapiro–Wilk test) and homogeneity of variances in different subpopulations. The flux data were assumed independent. Because of non-normally distributed data and/or data with unequal variances, the non-parametric Mann–Whitney rank sum test was run at p < 0.05 to test the statistical significance a) among flux rates from stems, shoots and forest floor; and b) between flux rates from dry and wet plots.
Correlation analyses (a) between stem, shoot and forest floor flux rates of N2O or CH4 and (b) between the trace gases flux rates and micro-climatic and other tree parameters were performed using non-parametric correlation analyses (Spearman’s rank correlation). The statistical significance was defined at p < 0.05.
SigmaPlot 11.0 (Systat Software, San Jose, California, USA) was used for statistical analyses.
Additional Information
How to cite this article: Machacova, K. et al. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci. Rep. 6, 23410; doi: 10.1038/srep23410 (2016).
References
Smith, K. A. et al. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur. J. Soil. Sci. 54, 779–791 (2003).
Bosse, U. & Frenzel, P. Activity and distribution of methane-oxidizing bacteria in flooded rice soil microcosms and in rice plants (Oryza sativa). Appl. Environ. Microb. 63, 1199–1207 (1997).
Butterbach-Bahl, K., Papen, H. & Rennenberg, H. Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ. 20, 1175–1183 (1997).
Yu, K. W., Wang, Z. P. & Chen, G. X. Nitrous oxide and methane transport through rice plants. Biol. Fert. Soils 24, 341–343 (1997).
Rusch, H. & Rennenberg, H. Black alder (Alnus glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 201, 1–7 (1998).
Nouchi, I., Mariko, S. & Aoki, K. Mechanism of methane transport from the rhizosphere to the atmosphere through rice plants. Plant Physiol. 94, 59–66 (1990).
McBain, M. C., Warland, J. S., McBride, R. A. & Wagner-Riddle, C. Laboratory-scale measurements of N2O and CH4 emissions from hybrid poplars (Populus deltoides × Populus nigra). Waste Manage. Res. 22, 454–465 (2004).
Pihlatie, M., Ambus, P., Rinne, J., Pilegaard, K. & Vesala, T. Plant-mediated nitrous oxide emissions from beech (Fagus sylvatica) leaves. New Phytol. 168, 93–98 (2005).
Machacova, K., Papen, H., Kreuzwieser, J. & Rennenberg, H. Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant Soil 364, 287–301 (2013).
Terazawa, K., Ishizuka, S., Sakata, T., Yamada, K. & Takahashi, M. Methane emissions from stems of Fraxinus mandshurica var. japonica trees in a floodplain forest. Soil Biol. Biochem. 39, 2689–2692 (2007).
Pangala, S. R., Gowing, D. J., Hornibrook, E. R. C. & Gauci, V. Controls on methane emissions from Alnus glutinosa samplings. New Phytol. 201, 887–896 (2014).
Pangala, S. R., Hornibrook, E. R. C., Gowing, D. J. & Gauci, V. The contribution of trees to ecosystem methane emissions in a temperate forested wetland. Glob. Change Biol. 21, 2642–2654 (2015).
Díaz-Pinés, E. et al. Nitrous oxide emissions from stems of ash (Fraxinus angustifolia Vahl) and European beech (Fagus sylvatica L.). Plant Soil. 10.1007/s11104-015-2629-8 (2015).
Mukhin, V. A. & Voronin, P. Y. Methanogenic activity of woody plants. Russ. J. Plant Physiol. 56, 138–140 (2009).
Mukhin, V. A. & Voronin, P. Y. Methane emission from living tree wood. Russ. J. Plant Physiol. 58, 344–350 (2011).
Covey, K. R., Wood, S. A., Warren, R. J., Lee, X. & Bradford, M. A. Elevated methane concentrations in trees of an upland forest. Geophys. Res. Lett. 39, 10.1029/2012GL052361 (2012).
Keppler, F., Hamilton, J. T. G., Brass, M. & Röckmann, T. Methane emissions from terrestrial plants under aerobic conditions. Nature 439, 187–191 (2006).
Vigano, I. et al. Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosciences 5, 937–947 (2008).
Smart, D. R. & Bloom, A. J. Wheat leaves emit nitrous oxide during nitrate assimilation. P. Natl. Acad. Sci. USA 98, 7875–7878 (2001).
Sundqvist, E., Crill, P., Mölder, M., Vestin, P. & Lindroth, A. Atmospheric methane removal by boreal plants. Geophys. Res. Lett. 39, 10.1029/2012GL053592 (2012).
Anderson, B. et al. Methane and Nitrous Oxide Emissions from Natural Sources. Office of Atmospheric Programs, EPA (United States Environmental Protection Agency), Washington D.C., USA (2010).
Ito, A. & Inatomi, M. Use of a process-based model for assessing the methane budgets of global terrestrial ecosystems and evaluation of uncertainty. Biogeosciences 9, 759–773 (2012).
Korhonen, J. F. J. et al. Nitrogen balance of a boreal Scots pine forest. Biogeosciences 10, 1083–1095 (2013).
Pihlatie, M. et al. Gas concentration driven fluxes of nitrous oxide and carbon dioxide in boreal forest soil. Tellus 59B, 458–469 (2007).
Dalal, R. C. & Allen, D. E. Turner Review No. 18: Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 56, 369–407 (2008).
Intergovernmental Panel on Climate Change. Climate Change 2013 The Physical Science Basis. (eds Stocker, T. F. et al. ), Cambridge University Press, Cambridge, UK (2013).
Butterbach-Bahl, K., Rothe, A. & Papen, H. Effect of tree distance on N2O and CH4-fluxes from soils in temperate forest ecosystems. Plant Soil 240, 91–103 (2002).
Von Arnold, K. et al. Can distribution of trees explain variation in nitrous oxide fluxes? Scand. J. Forest Res. 20, 481–489 (2005).
Dutaur, L. & Verchot, L. W. Global inventory of the soil CH4 sink. Global Biogeochem. Cy. 21, 10.1029/2006GB002734 (2007).
Sorz, J. & Hietz, P. Gas diffusion through wood: implications for oxygen supply. Trees 20, 34–41 (2006).
Hölttä, T. & Kolari, P. Interpretation of stem CO2 efflux measurements. Tree Physiol. 29, 1447–1456 (2009).
Pihlatie, M. et al. Methane fluxes in boreal forest soil. in Boreal Forest and Climate Change. (eds Hari, P. & Kulmala, L. ), Springer, The Netherlands, 393–398 (2008).
Ilvesniemi, H. & Liu, C. Biomass distribution in a young Scots pine stand. Boreal Environ. Res. 6, 3–8 (2001).
Hari, P. & Kulmala, M. Station for measuring ecosystem - atmosphere relations (SMEAR II). Boreal Environ. Res. 10, 315–322 (2005).
Pirinen, P. et al. Tilastoja Suomen Ilmastosta 1981–2010 (Climatological Statistics of Finland 1981–2010). Finnish Meteorological Institute Reports 2012/1, Helsinki, 1–96 (2012).
Ilvesniemi, H. et al. Long-term measurements of the carbon balance of a boreal Scots pine dominated forest ecosystem. Boreal Environ. Res. 14, 731–753 (2009).
Kolari, P. et al. CO2 exchange and component CO2 fluxes of a boreal Scots pine forest. Boreal Environ. Res. 14, 761–783 (2009).
Yassaa, N. et al. Diel cycles of isoprenoids in the emissions of Norway spruce, four Scots pine chemotypes and in boreal forest ambient air during HUMPPA-COPEC-2010. Atmos. Chem. Phys. 12, 7215–7229 (2012).
Altimir, N., Vesala, T., Keronen, P., Kulmala, M. & Hari, P. Methodology for direct field measurements of ozone flux to foliage with shoot chambers. Atmos. Environ. 36, 19–29 (2002).
Pihlatie, M. K. et al. Comparison of static chambers to measure CH4 emissions from soils. Agr. Forest Meteorol. 171–172, 124–136 (2013).
Repola, J., Ojansuu, R. & Kukkola, M. Biomass Functions for Scots Pine, Norway Spruce and Birch in Finland. Working Papers of the Finnish Forest Research Institute 53, Finnish Forest Research Institute, Helsinki, Finland (2007).
Mencuccini, M. & Bonosi, L. Leaf/sapwood area ratios in Scots pine show acclimation across Europe. Can. J. For. Res. 31, 442–456 (2001).
Levy, P. E. et al. Quantification of uncertainty in trace gas fluxes measured by the static chamber method. Eur. J. Soil Sci. 62, 811–821 (2011).
Urban, O. et al. Impact of clear and cloudy sky conditions on the vertical distribution of photosynthetic CO2 uptake within a spruce canopy. Funct. Ecol. 26, 46–55 (2012).
Ilvesniemi, H. et al. Water balance of a boreal Scots pine forest. Boreal Environ. Res. 15, 375–396 (2010).
Pumpanen, J. et al. Respiration in boreal forest soil as determined from carbon dioxide concentration profile. Soil Sci. Soc. Am. J. 72 (5), 1187–1196 (2008).
Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 3, 309–320 (1987).
Hölttä, T., Mäkinen, H., Nöjd, P., Mäkelä, A. & Nikinmaa, E. A physiological model of softwood cambial growth. Tree Physiol. 30, 1235–1252 (2010).
Altimir, N. et al. Foliage surface ozone deposition: a role for surface moisture? Biogeosciences 3, 209–228 (2006).
Acknowledgements
We would like to thank Hermanni Aaltonen for help with the gas chromatograph, Juho Aalto for help in installation of shoot chambers and calculation of stem and needle area, Teemu Paljakka for supplying us with sap flow data and Marian Pavelka for providing us with part of the vacuum pumps used and for his valuable advice regarding methodology. This research was financially supported by the EU FP7 project ExpeER (Grant Agreement no. 262060), the ENVIMET project (CZ.1.07/2.3.00/20.0246), NPU I project (LO1415), the Emil Aaltonen Foundation and Academy of Finland Research Fellow projects (263858, 288494), The Academy of Finland Centre of Excellence (project 1118615), ICOS-Finland (281255) and Helsinki University Centre for Environment, HENVI.
Author information
Authors and Affiliations
Contributions
K.M. had the idea for the study. K.M., M.P., A.V., P.K. and J.B. designed the study. K.M. carried out the field measurements and analysed the data. E.H., A.V., P.K., I.M. and J.P. provided data from ancillary measurements. K.M., J.B., A.V., E.H., P.K., I.M., J.P., M.A., O.U. and M.P. all contributed to writing the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Machacova, K., Bäck, J., Vanhatalo, A. et al. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci Rep 6, 23410 (2016). https://doi.org/10.1038/srep23410
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23410
This article is cited by
-
Variability in Stem Methane Emissions and Wood Methane Production of Different Tree Species in a Cold Temperate Mountain Forest
Ecosystems (2023)
-
Tree Foliage is a Methane Sink in Upland Temperate Forests
Ecosystems (2023)
-
Tree stem and soil methane and nitrous oxide fluxes, but not carbon dioxide fluxes, switch sign along a topographic gradient in a tropical forest
Plant and Soil (2023)
-
Soil-tree-atmosphere CH4 flux dynamics of boreal birch and spruce trees during spring leaf-out
Plant and Soil (2022)
-
Bark-dwelling methanotrophic bacteria decrease methane emissions from trees
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.