Abstract
Allocation of limited nutrients, such as nitrogen (N) and phosphorus (P), among plant organs reflects the influences of evolutionary and ecological processes on functional traits of plants and thus is related to functional groups and environmental conditions. In this study, we tested this hypothesis by exploring the stoichiometric scaling of N and P concentrations between twig stems and leaves of 335 woody species from 12 forest sites across eastern China. Scaling exponents of twig stem N (or P) to leaf N (or P) varied among functional groups. With increasing latitude, these scaling exponents significantly decreased from >1 at low latitude to <1 at high latitude across the study area. These results suggested that, as plant nutrient concentration increased, plants at low latitudes showed a faster increase in twig stem nutrient concentration, whereas plants at high latitudes presented a faster increase in leaf nutrient concentration. Such shifts in nutrient allocation strategy from low to high latitudes may be controlled by temperature. Overall, our findings provide a new approach to explore plant nutrient allocation strategies by analysing the stoichiometric scaling of nutrients among organs, which could broaden our understanding of the interactions between plants and their environments.
Similar content being viewed by others
Introduction
Allocation of limited nutrients, as an important strategy for plants in response to the changing environments, reflects the influences of evolutionary and ecological processes and trade-offs of multiple functions1,2,3,4. To maximize plant growth and maintain the optimal metabolic activities, plants need to balance the allocation of nutrients across organs under different stresses1,4,5,6,7. For example, plants under low soil nutrient availability often translocate nutrients from senesced tissues to new leaves because of the important carbon gain5. Plants under arid conditions generally allocate more N to leaves to compensate the low photosynthetic rate induced by the reducing stomatal conductance8,9. However, current knowledge of plant nutrient allocation strategies mainly comes from herbs, shrubs and tree seedlings1,7, with little knowledge from forest trees.
Exploring the associations of nutrient content across organs may be a way to reveal the allocation strategies of nutrients for tree species. From the ontogenetic perspective, plant nutrient contents across organs are tightly coordinated and their associations can be examined by scaling relationship analyses2,6,7,10. Scaling relationships among plant traits are widely studied, mainly in two ways: relationship of different traits within a specific organ and relationship of the same traits among organs. Within a specific organ, many scaling relationships are observed, such as leaf N vs. leaf P2,11,12,13, leaf lifespan vs. leaf mass per area14, nutrient content vs. photosynthetic rate12 and nutrient content vs. respiration rate15. For instance, the scaling exponent of leaf N to leaf P is <1, indicating a decline in leaf N:P with increasing plant growth rate, consistent with the “growth rate hypothesis”2,11,12,16. Among organs, previous studies focused on biomass partitioning17 and morphological linkages18,19,20. For example, Enquist and Niklas (2002)17 reported that leaf biomass scaled as the 3/4 power of stem biomass in seed plants, showing that plants allocate more biomass to stems than to leaves across a large scale. By contrast, the understanding of scaling relationships of nutrients among organs, especially for woody plants, is very limited.
Leaves of vascular plants play a crucial role in conducting photosynthesis, whereas twig stems provide mechanical support and transport water, carbohydrates and nutrients21,22,23. N and P allocated to leaves are the key components of metabolic and photosynthetic apparatuses24, whereas N and P allocated to twig stems play important roles in respiration15, internal nutrient recycling1,25 and photosynthate loading and export in the phloem24. Owing to the functional linkages between these two organs, nutrient concentrations between leaves and twig stems should also have tight associations via plant nutrient allocation strategies. Two recent studies revealed that scaling exponents of stem N (or P) to leaf N (or P) were >1, likely because plants required higher nutrient investments in stems (for photosynthate loading and export in the phloem24) than in leaves as plant nutrient increased2,7.
Like plant functional traits (e.g., leaf N, leaf P, leaf mass per area or leaf lifespan), the allocation of nutrients among organs can be considered as another plant trait that may reflect the interactions between plants and their environments per se. Previous studies indicate that functional groups, climate and soils have significant influences on plant functional traits and jointly determine their biogeographic patterns14,23,26,27,28,29,30. Therefore, we hypothesize that nutrient allocation among plant organs varies among functional groups and changes with environmental factors, causing a unique biogeographic pattern. Specifically, we hypothesize that: (1) as plant nutrient concentration increases, there is a faster increase in twig stem nutrient concentration for evergreen plants relative to deciduous plants and for legume relative to non-legume; (2) as plant nutrient concentration increases, plants at low latitudes show a faster increase in twig stem nutrient concentration, whereas plants at high latitudes show a faster increase in leaf nutrient concentration; and (3) both functional group and temperature together drive the latitudinal pattern of nutrient allocation, whereas soil nutrient availabilities and precipitation play modest roles in determining this pattern. In this study, we are to test these hypotheses by examining how functional groups, climate and soils jointly control the stoichiometric scaling of twig stem N (or P) to leaf N (or P) of 335 woody species from 12 forest sites in eastern China.
Results
Patterns of scaling exponents across functional groups
There were significant stoichiometric scaling relationships between twig stem N (or P, or N:P ratio) and leaf N (or P, or N:P ratio), but their scaling exponents varied among functional groups (Table 1; Fig. 1). Scaling exponents of twig stem P to leaf P (αP) and twig stem N:P ratio to leaf N:P ratio (αN:P) were highest in evergreen broad-leaved plants, followed by deciduous broad-leaved and coniferous plants (1.26, 0.96 and 0.70 for αP; 1.53, 0.89 and 0.62 for αN:P), whereas scaling exponent of twig stem N to leaf N (αN) was similar among the three functional types (1.20, 1.19 and 0.95). Legume species had higher scaling exponents than non-legume species (1.44 vs. 0.99 for αN; 1.86 vs. 0.88 for αP; 2.07 vs. 1.03 for αN:P).
Scaling relationships of twig stem N (or P, or N:P ratio) to leaf N (or P, or N:P ratio) for woody plants by functional group (deciduous/evergreen/conifer; legume/non-legume).
Reduced major axis (RMA) regression was used to determine the significant line (p < 0.05). All data were log10-transformed before analysis.
Relationships between scaling exponents and latitude/environmental factors
As latitude increased, scaling exponents for N and P and N:P ratio significantly decreased from >1 at low latitude (23.2°N) to <1 at high latitude (50.9°N) and this trend was consistent among forest types (i.e., tropical, temperate and boreal forest) (Fig. 2; Table S2 and S3). Along the latitudinal gradient, αN ranged from 1.45 at Mt. Dinghu with 23.2°N to 0.74 at Mt. Changbai with 42.1°N and αP varied from 1.36 at Mt. Dinghu to 0.71 at Mt. Genhe with 50.9°N and αN:P varied from 1.79 at Mt. Dinghu to 0.64 at Mt. Genhe with 50.9°N. Across the three biomes, αN, αP and αN:P were highest in tropical forests, followed by temperate and boreal forests (1.30, 0.97 and 0.89 for αN; 1.58, 0.97 and 0.80 for αP; 1.84, 1.19 and 0.74 for αN:P) (Fig. 2; Table S2). Moreover, both αN and αP were significantly correlated with mean annual temperature (MAT) (p < 0.05), but weakly correlated with annual precipitation (AP), soil total N (TN) and total P (TP) concentrations (Fig. 3). Specifically, scaling exponents increased with increasing MAT, ranging from 0.74 at −3.3 °C to 1.45 at 21 °C for αN and from 0.71 at −5.7 °C to 1.36 at 21 °C for αP. By contrast, αN:P was significantly correlated with MAT and AP and soil TP, but weakly correlated with soil TN (Fig. 3i–l).
The scaling exponents, αN, αP and αN:P, in tropical, temperate and boreal forests and along the latitudinal gradients in eastern China.
Points and error bars show the exponents and 95% confidence interval (CI). Different letters indicate significant difference (p < 0.05) based on a likelihood ratio test. Significant (p < 0.05) regression lines are fit to the exponents.
Discussion
The higher αP in evergreen broad-leaved species than in deciduous broad-leaved species (1.26 vs. 0.96 in Table 1) indicates that, as plant P increases, evergreen broad-leaved plants have a faster increase in twig stem P, whereas deciduous broad-leaved plants exhibit a faster increase in leaf P. Evergreen plants, distributed at low and mid-latitudes with low soil nutrient availability (Fig. S1)31, have evolved multiple adaptions to their environments, such as longer leaf lifespan, higher leaf mass per area and lower leaf photosynthetic rate than deciduous plants14,32,33,34. Compared with deciduous broad-leaved plants, evergreen broad-leaved plants might require more investments in phloem loading and export apparatus to meet the higher demand for photosynthate transport1,31, which is associated with increasing allocation of nutrients to twig stems. Additionally, their demand for nutrient storage via twig stems for a longer leaf lifespan might also induce a higher investment in twig stems than in leaves. In contrast, deciduous broad-leaved plants with short leaf lifespan need to maximize leaf photosynthetic activity to exploit light availabilities during the shorter growing season, resulting in more nutrient allocation to leaves. Furthermore, different from αP, αN is similar between evergreen broad-leaved plants and deciduous broad-leaved plants (1.20 vs 1.19 in Table 1, p > 0.05), which indicates that the two functional types perform the common allocation strategy of N between twig stem and leaf. We infer that, compared with N, plants tend to change their P allocation strategies more easily across the functional group.
For both αN and αP, legume species have higher values than non-legumes (1.44 vs. 0.99 for αN; 1.86 vs. 0.88 for αP) (Table 1), meaning that legume species allocate more N and P to twig stems as plant nutrient concentrations increase. Symbiotic N2-fixing bacteria in roots of legume species require much carbohydrate from photosynthetic tissues, because N acquisition through biological fixation has a higher energy cost than direct N absorption from the soil3,35. Thus, given the higher carbohydrate demand of symbiotic N2-fixing bacteria, legumes should increase the photosynthate loading and export rate in the phloem, which is associated with higher N and P investments in twig stems2.
Both αN and αP decrease from >1 to <1 with increasing latitude (Fig. 2), suggesting that, as plant nutrient concentration increased, plants at low latitudes tended toward a higher increase in twig stem nutrient concentration, whereas plants at high latitudes tended toward a higher increase in leaf nutrient concentration. This indicates a gradual shift in nutrient allocation strategy across a large geographic area. Moreover, plants at high latitudes had higher ratios of leaf N (or P) : twig stem N (or P) (Fig. 4a). These latitudinal patterns of scaling exponents (αN and αP) and nutrient ratios (leaf N/P : twig stem N/P) may be attributed to the changes in species composition from evergreen broad-leaved plants at low latitudes to deciduous broad-leaved and coniferous plants at high latitudes (Fig. S2)13, because αP was highest in evergreen broad-leaved plants, followed by deciduous broad-leaved and coniferous plants (1.26; 0.96; 0.70 in Table 1). However, the latitudinal pattern of αN was hardly explained by the changes in species composition because of similar αN across the three functional types. For evergreen broad-leaved plants, both αN and αP were higher in tropical forests than in temperate forests and for deciduous broad-leaved plants, both αN and αP were higher in temperate forests than in boreal forests (Fig. 5). Thus, changes in species composition may play a modest role in latitudinal patterns of nutrient allocation. Note that in this study we did not conduct detailed analyses on the scaling exponents of evergreen needle plants at boreal forests and deciduous plants at tropical forests and variations in the scaling exponents of coniferous plants among biomes because of the paucity of data and less representatives (Fig. 5; Fig. S2).
The scaling exponents, αN, αP and αN:P, for evergreen broadleaved plants in tropical and temperate forests (a) and for deciduous broadleaved plants in temperate and boreal forests (b)
Points and error bars show the exponent and 95% confidence interval (CI). Different letters indicate significant difference (p < 0.05) based on a likelihood ratio test.
Through the exploration of the relationships between scaling exponents and environmental factors, we found that MAT could be a main driver for these latitudinal patterns because of the tightest correlation between MAT and latitude (r2 = 0.94, p < 0.001 in Table S4). As the temperature increased, scaling exponents increased from <1 to >1, meaning that, as plant nutrient concentration increased, plants at low temperature exhibited a higher increase in leaf nutrient concentration, whereas a higher increase in twig stem nutrient concentration occurred for plants at high temperature. Plants at low temperature also had higher ratios of leaf N (or P) : twig stem N (or P) (Fig. 4b). Previous studies have revealed that temperature is the main driver of biogeographic patterns of leaf N and P concentration27,30,36 and biomass allocation37. According to the “temperature-plant physiological hypothesis”27, low temperature induces an increase in leaf nutrient concentrations to offset diminished efficiency of N-rich enzymes and P-rich RNA. However, compared with leaves, twig stems are less influenced by temperature, because their optimal functions are less dependent on nutrient compositions6. Thus, more nutrients allocated to leaves at low temperature are probably an adaptive strategy for plants to maintain the functional equilibrium for fitness. Furthermore, temperature largely drives the changes in growing season length and leaf lifespan34, which might influence plant nutrient allocation strategies. Decreasing temperature shortens the growing season length and leaf lifespan for deciduous broad-leaved plants34, hence more nutrients are allocated to leaves to maximize leaf functions for exploiting light resources during the shorter growing season.
In addition, although we did not detail the allocation of C concentrations, as showed in Fig. S3, the scaling exponents of twig stem C to leaf C concentration increased with MAT, suggesting that, as plant C concentration increased, plants at high temperature tended to a larger increase in twig stem C concentration. This further supports that a higher phloem loading and carbonhydrate transport in twig stems occurred for plants at high temperature, resulting in a higher increase in twig stem nutrient concentration.
Precipitation and soil nutrients might be potential drivers for these biogeographical patterns of nutrient allocation, considering their important roles in plant growth and biogeochemical cycling5. However, our study found that there were no significant relationships between scaling exponents and AP, soil TN and soil TP (Fig. 3). Eastern China exhibits a steeper thermal gradient than moisture gradient13. Given the high soil heterogeneity, soil TN and soil TP might not directly reflect the real nutrient availability for plants. In addition, these environmental factors vary collaterally with each other (Table S4) and their independent roles might be difficult to detect. Thus, future studies are needed to reveal their single effects on plant nutrient allocation strategies. Additionally, there was little impact of altitude on these patterns of nutrient allocation strategies because all scaling exponents showed no significant relationships with altitude (Fig. S4). Changes in the environmental conditions along the altitudinal gradient relative to latitudinal gradient were much smaller (Table S1) and thus the influence of altitude should be covered by that of latitude.
Plant N:P ratio is widely used as an indicator of N and P limitation in terrestrial ecosystem38. Schreeg et al. (2014)6 found that the scaling exponent of stem N:P ratio to leaf N:P ratio for tropical tree seedlings was >1, suggesting that stem N:P ratio might be a better indicator of soil nutrient availability. However, whether this could be applied to other species is little known. In this study, we found that αN:P varied among functional groups and along the latitudinal gradient. Woody plants at tropical forests show a higher variability of twig stem N:P ratios with αN:P > 1, whereas a more constrained twig stem N:P ratios with αN:P < 1 occurred for plants at boreal forests (Fig. 2f). Thus, whether twig N:P or leaf N:P is more sensitive to soil nutrient availability or other factors might depend on functional groups and sites.
Our findings suggest that functional groups and environmental factors jointly influence allocation of nutrients among organs and result in a gradual shift in nutrient allocation strategy from low to high latitudes across a large geographic scale in eastern China. Variations in the nutrient allocation across functional groups and environmental factors can be explained by the ‘optimal partitioning theory’ or ‘functional equilibrium concept’22,39, which means that plants would allocate their nutrients in an optimal way to obtain the ‘functional equilibrium’ for fitness. This large-scale pattern is more dependent upon temperature than other environmental factors (i.e., AP, soil TN and soil TP) according to our study, indicating that allocation strategy may also follow the “temperature-plant physiological hypothesis”27. Our results reveal that the scaling relationship is more “variable” than “invariant”, subject to the changes in plant nutrient allocation strategy with ambient environment. In general, by exploring the stoichiometric scaling of nutrients between leaves and twigs, we provide a new dimension to understand how plants regulate nutrient allocation strategies to adapt to ambient environment and thus would broaden our knowledge about the interactions between plants and their environments from a nutrient allocation perspective.
Materials and Methods
Study sites
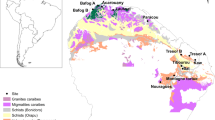
This study was conducted at 12 forest sites across eastern China with latitude from 18.7 °N to 50.9 °N (Fig. S1). Eastern China spans a large range of climates from cold and dry in the north to warm and moist in the south13 and has diverse vegetation types from boreal coniferous forest to tropical rainforest. Climate data, including mean annual temperature (MAT) and annual precipitation (AP), were obtained from local reports (Table S1). In the study area, MAT and AP ranges from −5.7 °C to 25.3 °C and from 423 mm to 2031 mm respectively and soil types shift from nutrient-rich brown soils to nutrient-poor tropical red soils. We classified these 12 sites into three biomes: boreal, temperate and tropical forests40. Here, tropical forests included forests situated at 18.7 °N and 23.2 °N, temperate forests included those situated between 29.8 °N and 42.4 °N, boreal forests included those situated between 45.3 °N and 50.9 °N.
Sampling and Measurement
We collected twig stem and leaf samples at 12 forest sites across eastern China, according to a previous protocol41. During the growing season (July–August), we chose dominant or common species at each site and then collected the fully expanded sun leaves from four or five individuals of each species. Accordingly, terminal 10–20 cm of twig stems (i.e., top twig stems) that supported the sun leaves were sampled. In total, we sampled 335 woody species in 198 genera and 73 families. Samples were dried at 60 °C to constant weight and then powdered using a ball mill (NM200, Retsch, Haan, Germany) before measuring N and P concentrations. Twig stem and leaf N concentrations were determined by the Dumas combustion method using an elemental analyser (2400 II CHS/O, Perkin-Elmer, USA). Twig and leaf P concentrations were determined using the molybdate/ascorbic acid method after H2SO4-HClO4 digestion42. For each site, we also collected three soil samples in three plots where these plants were sampled. A horizon samples of soils were randomly sampled and then thoroughly pooled for each plot to represent one soil sample. Soil samples were air-dried, sieved through a 2-mm mesh, handpicked to remove plant detritus and ground to pass through a 100-mesh sieve. We measured soil total N (TN) and total P (TP) concentrations using the same method for plant samples. Average values of three soil samples at the same site were calculated to represent the soil TN and TP concentration of each site.
Statistical analysis
Stoichiometric scaling relationships of twig stem N (or P, or N:P ratio) and leaf N (or P, or N:P ratio) were analysed using all original data of N and P concentrations and N:P mass ratios from individual plants (see Appendix S1 for details). Data were log10-transformed before analysis. RMA regression was used to determine the scaling function43, which was expressed by log10 Y = α*(log10 X) + β, where X and Y represent leaf N (or P, or N:P ratio) and twig stem N (or P, or N:P ratio) and α and β are the slope (i.e., scaling exponent) and intercept of regression line respectively. We performed these analyses in three ways. First, we divided all original data into respective functional groups (conifer/deciduous broad-leaved/evergreen broad-leaved; legume/non-legume) and three biomes (tropical/temperate/boreal forests) and then compared their scaling exponents. Second, we performed scaling analyses with original data for each site and then related scaling exponents to the latitude and environmental factors (MAT, AP, soil TN, soil TP) of each site using linear regressions. Third, we compared scaling exponents of evergreen broad-leaved plants from tropical and temperate forests and scaling exponents of deciduous broad-leaved plants from temperate and boreal forest. A likelihood ratio test was used to indicate the heterogeneity of RMA regression exponents among groups43. In addition, linear regressions were used to explore the relationships between log10-transformed ratios of leaf N (or P) : twig stem N (or P) and latitude and environmental factors. All statistical analyses were performed using R 2.15.244.
Additional Information
How to cite this article: Yan, Z. et al. Nutrient allocation strategies of woody plants: an approach from the scaling of nitrogen and phosphorus between twig stems and leaves. Sci. Rep. 6, 20099; doi: 10.1038/srep20099 (2016).
References
Bazzaz, F. A. & Grace, J. Plant resource allocation [143–158]. (Academic press, San Diego, 1997).
Kerkhoff, A. J., Fagan, W. F., Elser, J. J. & Enquist, B. J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 168, E103–E122 (2006).
Lambers, H., Chapin, F. S. & Pons, T. L. Plant physiological ecology [255–352]. (Springer-Verlag, New York, 2008).
Sardans, J. & Peñuelas, J. Tree growth changes with climate and forest type are associated with relative allocation of nutrients, especially phosphorus, to leaves and wood. Glob. Ecol. and Biogeogr. 22, 494–507 (2013).
Aerts, R. & Chapin, F. S. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv. Ecol. Res. 30, 1–67 (2000).
Schreeg, L. A., Santiago, L. S., Wright, S. J. & Turner, B. L. Stem, root and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 95, 2062–2068 (2014).
Yang, X. et al. Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across Northern China. Sci. Rep. 4 (2014).
Wright, I. J., Reich, P. B. & Westoby, M. Least-cost input mixtures of water and nitrogen for photosynthesis. Am. Nat. 161, 98–111 (2003).
Palmroth, S. et al. On the complementary relationship between marginal nitrogen and water-use efficiencies among Pinus taeda leaves grown under ambient and CO2-enriched environments. Ann. Bot. 111, 467–477 (2013).
Zhu, J. L., Shi, Y., Fang, L. Q., Liu, X. E. & Ji, C. J. Patterns and determinants of wood physical and mechanical properties across major tree species in China. Science China Life Sciences, 1–11 (2015).
Niklas, K. J., Owens, T., Reich, P. B. & Cobb, E. D. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 8, 636–642 (2005).
Reich, P. B. et al. Evidence of a general 2/3-power law of scaling leaf nitrogen to phosphorus among major plant groups and biomes. Proc. R. Soc. B Biol. Sci. 277, 877–883 (2010).
Han, W. X., Fang, J. Y., Reich, P. B., Ian Woodward, F. & Wang, Z. H. Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol. Lett. 14, 788–796 (2011).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Reich, P. B. et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 11, 793–801 (2008).
Sterner, R. W. & Elser, J. J. Ecological stoichiometry: the biology of elements from molecules to the biosphere [138–142]. (Princeton University Press, Princeton, 2002).
Enquist, B. J. & Niklas, K. J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 295, 1517–1520 (2002).
Westoby, M. & Wright, I. J. The leaf size–twig size spectrum and its relationship to other important spectra of variation among species. Oecologia 135, 621–628 (2003).
Sun, S. C., Jin, D. M. & Shi, P. L. The leaf size–twig size spectrum of temperate woody species along an altitudinal gradient: an invariant allometric scaling relationship. Ann. Bot. 97, 97–107 (2006).
Niklas, K. J. et al. “Diminishing returns” in the scaling of functional leaf traits across and within species groups. Proc. Natl Acad. Sci. USA 104, 8891–8896 (2007).
Pallardy, S. G. Physiology of woody plants [19–27]. (Academic Press, London, 2010).
Poorter, H. et al. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50 (2012).
Yao, F. Y. et al. Biogeographic patterns of structural traits and C: N: P stoichiometry of tree twigs in China’s forests. PLoS ONE 10, e0116391 (2015).
Marschner, H. & Marschner, P. Marschner’s mineral nutrition of higher plants [135–158]. (Academic press, London, 2012).
Marschnert, H., Kirkby, E. A. & Engels, C. Importance of cycling and recycling of mineral nutrients within plants for growth and development. Bot. Acta. 110, 265–273 (1997).
Reich, P. B., Walters, M. B. & Ellsworth, D. S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl Acad. Sci. USA 94, 13730–13734 (1997).
Reich, P. B. & Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl Acad. Sci. USA 101, 11001–11006 (2004).
Han, W. X., Fang, J. Y., Guo, D. L. & Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 168, 377–385 (2005).
Kattge, J. et al. TRY-a global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011).
Chen, Y. H., Han, W. X., Tang, L. Y., Tang, Z. Y. & Fang, J. Y. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 36, 178–184 (2013).
Givnish, T. J. Adaptive significance of evergreen vs. deciduous leaves: solving the triple paradox. Silva Fenn. 36, 703–743 (2002).
Takashima, T., Hikosaka, K. & Hirose, T. Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 27, 1047–1054 (2004).
Wright, I. J. et al. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 14, 411–421 (2005).
van Ommen Kloeke, A. E. E., Douma, J. C., Ordoñez, J. C., Reich, P. B. & Van Bodegom, P. M. Global quantification of contrasting leaf life span strategies for deciduous and evergreen species in response to environmental conditions. Glob. Ecol. Biogeogr. 21, 224–235 (2012).
Vitousek, P. M. & Field, C. B. Ecosystem constraints to symbiotic nitrogen fixers: a simple model and its implications. Biogeochemistry 46, 179–202 (1999).
Kerkhoff, A. J., Enquist, B. J., Elser, J. J. & Fagan, W. F. Plant allometry, stoichiometry and the temperature‐dependence of primary productivity. Glob. Ecol. Biogeogr. 14, 585–598 (2005).
Reich, P. B. et al. Temperature drives global patterns in forest biomass distribution in leaves, stems and roots. Proc. Natl Acad. Sci. USA 111, 13721–13726 (2014).
Güsewell, S. N. : P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266 (2004).
Gedroc, J. J., McConnaughay, K. D. M. & Coleman, J. S. Plasticity in root/shoot partitioning: optimal, ontogenetic, or both? Funct. Ecol. 10, 44–50 (1996).
Yuan, Z. Y., Chen, H. Y. H. & Reich, P. B. Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nature commun. 2, doi: 10.1038/ncomms1346 (2011).
Cornelissen, J. H. C. et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380 (2003).
John, M. K. Colorimetric determination of phosphorus in soil and plant materials with ascorbic acid. Soil Sci. 109, 214–220 (1970).
Warton, D. I., Wright, I. J., Falster, D. S. & Westoby, M. Bivariate line‐fitting methods for allometry. Biol. Rev. Camb. Philos. Soc. 81, 259–291 (2006).
R Development Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, 2012).
Acknowledgements
We thank B. Zhu, E.Z. Du and C.J. Ji for helpful suggestions on this manuscript. We also thanks L.Y. Tang, L.P. L.I. and J.Y. Sun for their assistance in field work. The research was supported by the National Natural Science Foundation of China (Project Nos. 31321061, 31330012 and 41173083).
Author information
Authors and Affiliations
Contributions
J.F., Z.Y. and P.L. conceived the research. Both Y.C. and Z.Y. collected and analyzed the data. Z.Y., L.P., W.H. and J.F. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Yan, Z., Li, P., Chen, Y. et al. Nutrient allocation strategies of woody plants: an approach from the scaling of nitrogen and phosphorus between twig stems and leaves. Sci Rep 6, 20099 (2016). https://doi.org/10.1038/srep20099
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep20099
This article is cited by
-
Nutrient allocation patterns of Picea crassifolia on the eastern margin of the Qinghai-Tibet Plateau
International Journal of Biometeorology (2024)
-
Spatial characteristics of nutrient allocation for Picea crassifolia in soil and plants on the eastern margin of the Qinghai-Tibet Plateau
BMC Plant Biology (2023)
-
Does nitrogen fertilization alter the scaling relationships of multinutrients in tree organs? Evidence from Chinese hickory (Carya cathayensis) saplings
Plant and Soil (2023)
-
Effects of Nitrogen and Phosphorus Limitation on the Growth and Phosphorus Allocation of Alfalfa (Medicago sativa L.)
Journal of Soil Science and Plant Nutrition (2023)
-
Clones of non-pathological witches’ broom and normal crown from the same trees in Pinus sibirica on common and separate rootstocks: how and why David wins over Goliath
New Forests (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.