Abstract
Recent experiments showed that some perovskite oxyhydrides have surprisingly high magnetic-transition temperature. In order to unveil the origin of this interesting phenomenon, we investigate the magnetism in SrCrO2H and SrVO2H on the basis of first-principles calculations and Monte Carlo simulations. Our work indicates that the Cr-O-Cr superexchange interaction in SrCrO2H is unexpectedly strong. Different from the previous explanation in terms of the H− ion substitution induced increase of the Cr-O-Cr bond angle, we reveal instead that this is mainly because the 3d orbitals in perovskite oxyhydrides becomes more delocalized since H− ions have weaker electronegativity and less electrons than O2− ions. The delocalized 3d orbitals result in stronger Cr-O interactions and enhance the magnetic-transition temperature. This novel mechanism is also applicable to the case of SrVO2H. Furthermore, we predict that SrFeO2H will have unprecedented high Neel temperature because of the extraordinarily strong Fe-H-Fe σ-type interactions. Our work suggests the anion substitution can be used to effectively manipulate the magnetic properties of perovskite compounds.
Similar content being viewed by others
Introduction
Complex transition metal oxides have been the subject of enduring interest due to the wide variety of physical properties they exhibit, to name a few, high-Tc superconductivity, magnetoresistance, multiferroicity, thermoelectric response and so on1,2,3,4,5,6,7. For the past few years, scientists have found that replacing segmental O2− ions in transition metal oxides by N3−, F− or S2− can result in novel materials such as pigments, water-splitting photocatalysis, dielectric and cathode material8,9,10,11,12. Different from N3−, F− or S2− which has p valence electrons, the H− anion has a filled 1 s2 electronic configuration that is fundamentally different from the O2− ion case. Therefore, it is expected that the H− ion substitution might leads to exotic behaviors in perovskite. In pioneering works, a large amount of hydrogen species were incorporated into ATiO3 (A = Ba, Sr, Ca) and Sr2VO4 lattice through the use of CaH2 reductant13,14,15,16. The resulting oxyhydride ATiO3−xHx exhibits high electronic conductivity and its hydride ions are exchangeable with gaseous hydrogen at elevated temperature, indicating that it can be an ideal mixed electron/hydride proton conductor for electrochemical applications13,14,15. In oxyhydride Sr2VO4−xHx, the hydride ion could act as an effective carrier dopant because the hydrogen and oxygen concentrations can be controlled16.
Interestingly, it was experimentally found that the magnetic properties of transition metal oxides may change dramatically if some oxygen anions are replaced by hydrogen anions. An antiferromagnetic-to-ferromagnetic transition in EuTiO3–xHx induced by hydride substitution was reported, where the ferromagnetism was caused by the Ruderman–Kittel–Kasuya–Yosida (RKKY) interaction between the Eu2+ spins mediated by the itinerant Ti 3d electrons17. LaSrCoO3H0.718,19,20 and Sr3Co2O4.33H0.8421 were found to display high magnetic transition temperatures. Recently, the stoichiometric perovskite oxyhydrides SrCrO2H and SrVO2H have been synthesized22,23. The average structure of SrCrO2H is the cubic perovskite where the hydride ions are randomly distributed. In SrVO2H, the planar VO2 layers are connected by hydride ions. The experimentally observed antiferromagnetic (AFM) Neel temperature (TN) of SrCrO2H and SrVO2H are around 380 K and higher than 300 K22,23, respectively. It is puzzling that the Neel temperature TN of SrCrO2H is higher than that (290 K)24,25 of LaCrO3. In both SrCrO2H and LaCrO3, the valence of Cr element is 3+ with the three 3d electrons occupying the t2g orbitals, that is, the electron configuration is (dxy)1(dyz)1(dxz)1. In average, each Cr3+ ion in SrCrO2H has two Cr-H-Cr superexchange paths and four Cr-O-Cr superexchange paths. Since the t2g orbitals of the Cr3+ ion could not interact with the 1s orbitals of H− ions by symmetry, the Cr-H-Cr superexchange interaction is negligible. In LaCrO3, there are six Cr-O-Cr superexchange paths for each Cr3+ ion. So the fact that the Neel temperature TN of SrCrO2H is higher than that of LaCrO3 is rather unexpected.
To probe the origin of the high TN in SrCrO2H and SrVO2H, we studied the magnetic properties of SrCrO2H, SrVO2H and LaCrO3 based on the density functional theory (DFT). We show that due to weaker electronegativity and less electrons of H− ions than those of O2− ions, the substitution of H− ions with O2− ions lead to more delocalized 3d orbitals of Cr3+ ions which make Cr-O-Cr superexchange in SrCrO2H much stronger and leads to a high TN. This new mechanism is also applicable to the case of SrVO2H.
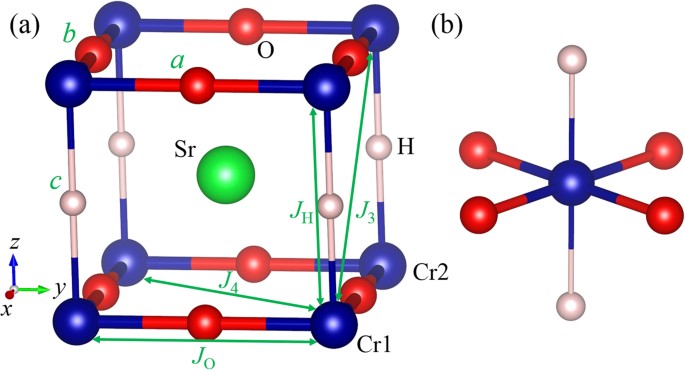
Although the H− ion was experimentally reported to be randomly distributed in SrCrO2H, we will adopt an ordered structure (see Fig. 1a) to investigate the magnetism in SrCrO2H for the following reasons. First, by using the cluster expansion approach, we find the ground state structure of SrCrO2H is similar to the experimentally observed structure of SrVO2H. Second, we find that the magnetic properties of another SrCrO2H structure with a more random H− ion distribution are similar to those of the ordered structure (see Supplementary Material). The ground state structure (tetragonal P4/mmm symmetry) of SrCrO2H has one H− ion and two O2− ions in the primitive cell. The Cr3+ cations are located within square planes of oxide ions and form CrO2 sheets. The CrO2 sheets are connected by hydride ions, which occupy the remaining two coordination sites around each Cr3+ center. Thus the ground state structure of SrCrO2H can be described by a CrO2-SrH-CrO2-SrH stacking sequence. The computed phonon frequencies26 (see Supplementary Figure S3) indicate that the ground state structure of SrCrO2H is dynamically stable.
(a) PDOS of the spin-up Cr3+ ion in SrCrO2H with the G-type AFM order. (b) Energy level and electron occupation for the spin-up Cr1 ion and spin-down Cr2 ion. The positions of Cr1 and Cr2 are shown in Fig. 1a. The spin-up and spin-down levels are denoted by black and blue colors, respectively. The effective orbitals hoppings responsible for the Cr1-Cr2 superexchange interaction are illustrated.
To examine the magnetic properties of SrCrO2H, we considered four ordered spin states, namely, the ferromagnetic (FM), A-type AFM, C-type AFM and G-type AFM states. The experimentally observed AFM structure is the G-type. Our GGA + U calculations show that all the AFM states have lower energy than the FM state and the G-type AFM is indeed the ground state, in consistent with experimental observations22. To extract the values of the spin exchange parameters, we adopt the four-state mapping approach27,28. There are four relevant Cr-Cr spin exchange interactions (see Fig. 1a): (1) JO is the nearest neighbor (NN) superexchange interaction for the Cr-O-Cr path; (2) JH is the NN spin exchange interaction for the Cr-H-Cr path; (3) J3 is next nearest neighbor (NNN) super-superexchange interaction in the plane; (4) J4 is the NNN out of plane super-superexchange interaction. The calculated exchange parameters in the tetragonal structure of SrCrO2H are summarized in Table 1. As expected, the NN Cr-O-Cr path has the strongest spin exchange interaction (JO) since it is mediated by the strong π-π hybridization between 3d and 2p orbitals. The NN spin exchange interaction JH is weakly AFM due to the direct through-space overlap between the t2g orbitals of Cr3+ ions. The NNN exchange interactions (J3 and J4) are negligible. The AFM nature of the NN interactions can result in the G-type AFM order. Based on the calculated spin exchange parameters, our Monte Carlo (MC) simulations indicate that the TN is around 285 K. If a smaller Hubbard U is adopted, we can get higher TN according to the theory of superexchange, in better agreement with the experimentally observed TN22.
For comparison, we also studied the magnetism in LaCrO3. Experiments show the ground state structure of LaCrO3 is the GdFeO3–type distorted perovskite with Pbnm space group and its lattice constants are a = 5.478, b = 7.759 and c = 5.51629. The lattice constants of our optimized Pbnm structure are a = 5.468 Å, b = 7.758 Å and c = 5.497 Å, in good agreement with experiment. The obtained spin exchange parameters of the optimized LaCrO3 are summarized in Table 1. The NN interaction JO is AFM and the NNN interactions are negligible. This is in accord with the experimentally observed G-type AFM ground state in LaCrO3. Compared with SrCrO2H, it is clear that JO in LaCrO3 is much weaker. Based on the calculated spin exchange parameters, our MC simulations indicate that the TN for LaCrO3 is around 133 K. Therefore, our theoretical calculations confirm the experimental observation that SrCrO2H has a higher TN than LaCrO3, as shown in Fig. 3.
Now we begin to understand the difference in the magnetic properties between SrCrO2H and LaCrO3. There are two significant differences between SrCrO2H and LaCrO3. First, there is a structural difference. The CrO6 octahedron in LaCrO3 is tilted due to a small tolerance factor, while there is no octahedron tilt in SrCrO2H. As a result, the Cr-O-Cr angle in SrCrO2H is 180° while the average Cr-O-Cr angle in LaCrO3 is 167°. Second, a chemical difference exists since one third of the O2− ions are replaced by H− ions.
To make clear whether the structural difference or the chemical difference is responsible for the stronger Cr-O-Cr exchange in SrCrO2H, we investigate the magnetic properties of SrCrO2H and LaCrO3 with the same cubic perovskite crystal structure. The lattice constant is set to be the average lattice constant (3.85 Å) of experimental SrCrO2H structure22. Note that the hypothetic cubic LaCrO3 phase can be regarded as a result of substituting Sr2+ and H− in cubic SrCrO2H with La3+ and O2−, respectively. The computed spin exchange parameters of these two cubic structures are summarized in Table 1. Surprisingly and interestingly, the Cr-O-Cr superexchange (29.69 meV) in SrCrO2H is almost as twice as that (14.25 meV) in LaCrO3 despite of the fact that the Cr-O bond length and the Cr-O-Cr bond angle are identical in cubic SrCrO2H and LaCrO3. As expected, the Cr-O-Cr exchange in cubic LaCrO3 is stronger than that (8.88 meV) in Pbnm LaCrO3 according to the Goodenough-Kanamori rule30,31,32. Previously, it was suggested that the structural difference is solely responsible for the high TN in SrCrO2H22. However, our calculations show that the structure difference and chemical difference enhance the Cr-O-Cr exchange interaction by 5.37 meV and 15.44 meV, respectively. Therefore, the effect of chemical difference on the Cr-Cr exchange interaction is much more important than that of the structural difference.
To account for why JO in the cubic SrCrO2H is as twice as that in the cubic LaCrO3, we examine their electronic structures in details. Figure 2a shows the partial density of states (PDOS) of the spin-up Cr3+ ion in SrCrO2H with the G-type AFM order. We can see that, for SrCrO2H, the majority-spin t2g (dxy, dxz and dyz) orbitals are occupied by three electrons but the majority-spin eg orbitals are unoccupied, which is similar to the orbital occupation in LaCrO3. The energy level and occupancy of the 3d orbitals of the tetragonal SrCrO2H are shown in Fig. 2b. Note that the t2g orbitals split into the low-lying two-fold degenerate dxz/dyz level and a higher dxy level as a result of the tetragonal symmetry.
Without loss of generality, we consider the spin exchange JO for the Cr1-O-Cr2 path along the x axis, as shown in Fig. 1a. According to Anderson’s superexchange theory33, the magnitude of the spin exchange can be estimated by  , where t is the effective hopping between the d orbitals and Δ is the energy difference between majority-spin and minority-spin orbitals. In the cubic SrCrO2H or LaCrO3, the dxz orbital can only interact with the neighboring dxz orbital and so do dxy, dyz. The magnitude of JO for the Cr1-O-Cr2 path can be estimated as
, where t is the effective hopping between the d orbitals and Δ is the energy difference between majority-spin and minority-spin orbitals. In the cubic SrCrO2H or LaCrO3, the dxz orbital can only interact with the neighboring dxz orbital and so do dxy, dyz. The magnitude of JO for the Cr1-O-Cr2 path can be estimated as  , where tm (m=xz, yz, xy) is effective hopping between the m orbitals of Cr1 and Cr2 and Δm is the energy difference between the majority-spin m orbital and minority-spin m orbital of the Cr3+ ion (see Fig. 2b). The Δm parameters are estimated by constructing the maximally localized Wannier functions (MLWFs) based on the ferromagnetic electronic structure. It turns out that Δm (about 5.3 eV) in SrCrO2H is rather close to that (about 5.5 eV) in LaCrO3. Therefore, we can regard Δm as a constant. The effective hopping paramters tm between the Cr 3d orbitals is obtained by constructing the MLWFs using the spin-unpolarized Bloch wavefunctions. These hopping parameters are listed in Table 2. We can see that the hopping parameter tyz between the dyz orbitals of Cr1 and Cr2 is negligible since these two orbitals are almost parallel to each other. The π-π hopping parameter txy in the cubic SrCrO2H are the same as that in the cubic LaCrO3. The striking result is that the π-π hopping parameter txz between the dxz orbitals of Cr1 and Cr2 in the cubic SrCrO2H is almost 50% stronger than that in LaCrO3. Using these hopping parameters, we can estimate the ratio between JO in cubic SrCrO2H and that in LaCrO3 as:
, where tm (m=xz, yz, xy) is effective hopping between the m orbitals of Cr1 and Cr2 and Δm is the energy difference between the majority-spin m orbital and minority-spin m orbital of the Cr3+ ion (see Fig. 2b). The Δm parameters are estimated by constructing the maximally localized Wannier functions (MLWFs) based on the ferromagnetic electronic structure. It turns out that Δm (about 5.3 eV) in SrCrO2H is rather close to that (about 5.5 eV) in LaCrO3. Therefore, we can regard Δm as a constant. The effective hopping paramters tm between the Cr 3d orbitals is obtained by constructing the MLWFs using the spin-unpolarized Bloch wavefunctions. These hopping parameters are listed in Table 2. We can see that the hopping parameter tyz between the dyz orbitals of Cr1 and Cr2 is negligible since these two orbitals are almost parallel to each other. The π-π hopping parameter txy in the cubic SrCrO2H are the same as that in the cubic LaCrO3. The striking result is that the π-π hopping parameter txz between the dxz orbitals of Cr1 and Cr2 in the cubic SrCrO2H is almost 50% stronger than that in LaCrO3. Using these hopping parameters, we can estimate the ratio between JO in cubic SrCrO2H and that in LaCrO3 as:

Thus, our first-principles result can be well accounted for by this simple model. This analysis clearly shows that the stronger txz hopping in SrCrO2H is responsible for its high TN.
Figure 4a,b shows the real-space distribution of the dxz-like MLWFs in the cubic SrCrO2H and LaCrO3. It is clear that the effective dxz-like MLWF not only distributes around the Cr ion, but also has tails on the neighboring O2− ions due to the anti-bonding π* hybridization between Cr-dxz and O-2p orbitals. It is the tails on the O2− ions that mediate the effective hopping between the Cr-dxz orbitals. An interesting observation is that the lobes on the O2− ion in the MLWF of SrCrO2H are bigger than those in LaCrO3. This suggests that the interaction between Cr-dxz orbital and O-pz orbital in SrCrO2H is stronger than that in LaCrO3, in agreement with our previous result that the effective hopping between the dxz orbitals of Cr1 and Cr2 in SrCrO2H is larger than that in LaCrO3.
Isosurface plots of the Cr-dxz like MLWFs for (a) cubic SrCrO2H and (b) cubic LaCrO3.
The lobes on the O2− ion in the MLWF of SrCrO2H are bigger than those of LaCrO3, indicating that the hybridization between Cr-dxz orbital and O-pz is stronger in SrCrO2H. Contour plots of the electrostatic potential in (c) cubic SrCrO2H and (d) cubic LaCrO3, projected on the xz-plane passing through Cr, H and O sites. The electrostatic potential along the Cr-H direction is much weaker than that along the Cr-O direction in SrCrO2H.
We propose that the stronger interaction between Cr-dxz orbital and O-pz orbital in SrCrO2H results from the more delocalized Cr-dxz orbital in SrCrO2H (see Supplementary Figure S5). This is supported by a separate MLWF analysis which indicates that the spread of the atomic Cr-dxz orbital in the cubic SrCrO2H is larger than that in the cubic LaCrO3. The more delocalized Cr-dxz orbital in SrCrO2H can be reasoned by considering the electrostatic potential exerted on the Cr 3d electrons. The contour plots of the electrostatic potential on the x-z plane are displayed in the Fig. 4c,d. We can see that the electrostatic potential along the Cr-H direction is much weaker than that along the Cr-O direction. Thus, the dxz orbital in SrCrO2H is more delocalized along the z-axis, as can also be seen from Fig. 4a. It is the weaker repulsion between the H− ion and the 3d electrons that makes the dxz orbital in SrCrO2H more delocalized. The weaker electrostatic potential along the Cr-H direction results from the fact that H− ions have the weaker electronegativity and less charge than O2− ions. Therefore, the replacement of O2− ions by H− ions will not only change the hybridization type between the transition metal and the anions, but also affect the wavefunction distribution of d orbitals. Such novel mechanism revealed here for SrCrO2H can be also applied to SrVO2H. The only difference is that there is one d electron less than that in SrCrO2H, which makes the TN slightly lower (see Supplementary Material). We note that first-principles calculations34 were recently carried out to study the electronic and magnetic properties of SrVO2H. However, the mechanism for the high Neel temperature in SrVO2H was not discovered.
The mechanism that the H− ion induced delocalization of the d orbitals is general and may have profound effect on the electronic and magnetic properties of other perovskite oxyhydrides. Below we will predict that SrFeO2H has an extremely high TN. With the cluster expansion approach, we predict that SrFeO2H takes the same ground state structure as that of SrCrO2H. This is reasonable since the ionic radius of the Fe3+ ion is close to that of the Cr3+ ion. The optimized lattice constants a and c for SrFeO2H are 3.997 Å and 3.645 Å, respectively. The computed phonon dispersion26 (see Supplementary Figure S4) indicates that SrFeO2H is dynamically stable. The spin exchange parameters calculated for the optimized SrFeO2H structures are listed in Table 1. SrFeO2H takes the G-type AFM order as the magnetic ground state since the NN AFM spin exchanges JO (Fe-O-Fe) and JH (Fe-H-Fe) are dominant. To our surprise, the spin exchange JH (89.26 meV) in SrFeO2H is much stronger than JO (39.63 meV) in SrFeO2H. Note that the much stronger Fe-H-Fe interaction is not mainly caused by the shorter Fe-H distance than the Fe-O distance, because similar results are also obtained in the cubic perovskite SrFeO2H structure. In fact, the much stronger Fe-H-Fe interaction is mainly because the out-of-plane Fe- orbital is more delocalized than the in-plane Fe-
orbital is more delocalized than the in-plane Fe- and
and  orbitals. Therefore, the σ bond between the H 1s orbital and Fe-
orbitals. Therefore, the σ bond between the H 1s orbital and Fe- orbital is much stronger than the σ bond between the O-2p orbitals and the in-plane Fe
orbital is much stronger than the σ bond between the O-2p orbitals and the in-plane Fe  /
/ orbitals (see Supplementary Figure S6). Similar to the cases of SrCrO2H and SrVO2H, the weaker electrostatic potential of H− ions exerting on the d electrons of Fe3+ ions leads to more delocalized Fe-
orbitals (see Supplementary Figure S6). Similar to the cases of SrCrO2H and SrVO2H, the weaker electrostatic potential of H− ions exerting on the d electrons of Fe3+ ions leads to more delocalized Fe- orbitals, which results in an anomalously strong spin exchange JH. Our MC simulations indicate that the TN of SrFeO2H is around 826 K, which is even higher than that of BiFeO3 (TN = 643 K)35 and SrFeO2 with a quasi-two-dimensional structure (TN = 473 K)36,37. Our result suggests that the replacement of O2− ions by H− ions can enhance the magnetic interactions not only in t2g d2 and d3 systems, but also in d5 systems. Our work suggests that the high magnetic transition temperature in LaSrCoO3H0.718,19,20 and Sr3Co2O4.33H0.8421 should be also due to the H− ion induced delocalization of the 3d eg orbitals.
orbitals, which results in an anomalously strong spin exchange JH. Our MC simulations indicate that the TN of SrFeO2H is around 826 K, which is even higher than that of BiFeO3 (TN = 643 K)35 and SrFeO2 with a quasi-two-dimensional structure (TN = 473 K)36,37. Our result suggests that the replacement of O2− ions by H− ions can enhance the magnetic interactions not only in t2g d2 and d3 systems, but also in d5 systems. Our work suggests that the high magnetic transition temperature in LaSrCoO3H0.718,19,20 and Sr3Co2O4.33H0.8421 should be also due to the H− ion induced delocalization of the 3d eg orbitals.
In summary, we perform a systematic theoretical study on the magnetic properties of perovskite oxyhydrides. The high magnetic transition temperature in SrCrO2H is revealed to be due to the delocalization of 3d orbitals in perovskite oxyhydrides. This is because H− ions have weaker electronegativity and fewer electrons than O2− ions. The more delocalized 3d orbitals in SrCrO2H make Cr-O-Cr superexchange strong and TN high. This novel mechanism also applies to the case of SrVO2H. We predict that the σ-type Fe-H-Fe interactions in SrFeO2H are extraordinarily strong which also result from the delocalization of the 3d orbitals. The delocalization of d orbitals in oxyhydrides discovered in this work is universal and may also have profound effects on properties other than the magnetic properties.
Methods
Our DFT calculations are performed on the basis of the projector augmented wave method38,39 encoded in the Vienna ab initio simulation package40,41 (VASP) using the generalized-gradient approximation (GGA) of Perdew, Burke and Ernzerhof42. The plane-wave cutoff energy is set to be 450 eV. To properly describe the strong electron correlation in the 3d transition-metal oxide, the GGA plus on-site repulsion U method (GGA + U) is employed43. U = 4 eV and J = 1eV are applied to the 3d electron of Cr3+ ions. The maximally localized Wannier functions (MLWFs) are constructed with the Wannier90 program44,45. The spread functional is considered to be converged if the corresponding fractional change because two successive iterations is smaller than 10−10. To find the ground state structures of SrCrO2H, SrVO2H and SrFeO2H, we adopt the cluster expansion approach46 by using the alloy theoretic automation toolkit (ATAT)47.
We perform parallel tempering Monte Carlo (PTMC) simulations48,49 to estimate the magnetic transition temperature. In PTMC simulations, many replicas with different temperature are simultaneously simulated and a virtual process exchanging configuration of these replicas is introduced. PTMC simulations can avoid a local minimum at low temperatures and can reduce relaxation time. We adopt a 10 × 10 × 10 supercell to perform PTMC simulations. Our test shows that the results obtained with a 12 × 12 × 12 supercell are almost the same as those with the 10 × 10 × 10 supercell. The number of replicas is set to 120.
Additional Information
How to cite this article: Liu, K. et al. Orbital Delocalization and Enhancement of Magnetic Interactions in Perovskite Oxyhydrides. Sci. Rep. 6, 19653; doi: 10.1038/srep19653 (2016).
References
Okuda, T., Nakanishi, K., Miyasaka, S. & Tokura, Y. Large Thermoelectric Response of Metallic Perovskites: Sr1−xLaxTiO3 (0 ≤ x ≤ 0.1). Phys. Rev. B 63, 113104 (2001).
Dagotto, E., Hotta, T. & Moreo, A. Colossal Magnetoresistant Materials: The Key Role of Phase Separation. Phys. Rep. 344, 1–153 (2001).
Moritomo, Y., Asamitsu, A., Kuwahara, H. & Tokura, Y. Giant Magnetoresistance of Manganese Oxides with a Layered Perovskite Structure. Nature 380, 141–144 (1996).
Vonhelmolt, R., Wecker, J., Holzapfel, B., Schultz, L. & Samwer, K. Giant Negative Magnetoresistance in Perovskitelike La2/3Ba1/3MnOx Ferromagnetic Film. Phys. Rev. Lett. 71, 2331–2333 (1993).
Bednorz, J. G. & Muller, K. A. Possible High Tc Superconductivity in the Ba-La-Cu-O System. Z. Phys. B: Condens. Matter 64, 189–193 (1986).
Cohen, R. E. Origin of Ferroelectricity in Perovskite Oxides. Nature 358, 136–138 (1992).
Johnston, D. C., Prakash, H., Zachariasen, W. H. & Viswanathan, R. High Temperature Superconductivity in the Li-Ti-O Ternary System. Mater. Res. Bull. 8, 777–784 (1973).
Jansen, M. & Letschert, H. P. Inorganic Yellow-Red Pigments without Toxic Metals. Nature 404, 980–982 (2000).
Kasahara, A. et al. Photoreactions on LaTiO2N under Visible Light Irradiation. J. Phys. Chem. A 106, 6750–6753 (2002).
Zhang, Y. R., Masubuchi, Y., Motohashi, T., Kikkawa, S. & Hirota, K. Hot Isostatic Press Sintering and Dielectric Properties of SrTaO2N Ceramics. Ceram. Int. 39, 3377–3380 (2013).
Zhang, Y.-R., Motohashi, T., Masubuchi, Y. & Kikkawa, S. Sintering and Dielectric Properties of Perovskite SrTaO2N Ceramics. J. Eur. Ceram. Soc. 32, 1269–1274 (2012).
Tong, W., Yoon, W. S., Hagh, N. M. & Amatucci, G. G. A Novel Silver Molybdenum Oxyfluoride Perovskite as a Cathode Material for Lithium Batteries. Chem. Mater. 21, 2139–2148 (2009).
Kobayashi, Y. et al. An Oxyhydride of BaTiO3 Exhibiting Hydride Exchange and Electronic Conductivity. Nat. Mater. 11, 507–511 (2012).
Sakaguchi, T. et al. Oxyhydrides of (Ca,Sr,Ba)TiO3 Perovskite Solid Solutions. Inorg. Chem. 51, 11371–11376 (2012).
Yajima, T. et al. Epitaxial Thin Films of ATiO3−xHx (A = Ba, Sr, Ca) with Metallic Conductivity. J. Am. Chem. Soc. 134, 8782–8785 (2012).
Bang, J. et al. Hydrogen Ordering and New Polymorph of Layered Perovskite Oxyhydrides: Sr2VO4−xHx . J. Am. Chem. Soc. 136, 7221–7224 (2014).
Yamamoto, T. et al. An Antiferro-to-Ferromagnetic Transition in EuTiO3−xHx Induced by Hydride Substitution. Inorg. Chem. 54, 1501–1507 (2015).
Blundell, S. J. et al. Magnetism in Oxide Chains Bridged with the Hydride Anion: LaSrCoO3H0.7 Studied Using Muon-Spin Rotation. Physica B 326, 527–531 (2003).
Bridges, C. A., Darling, G. R., Hayward, M. A. & Rosseinsky, M. J. Electronic Structure, Magnetic Ordering and Formation Pathway of the Transition Metal Oxide Hydride LaSrCoO3H0.7 . J. Am. Chem. Soc. 127, 5996–6011 (2005).
Hayward, M. A. et al. The Hydride Anion in an Extended Transition Metal Oxide Array: LaSrCoO3H0.7 . Science 295, 1882–1884 (2002).
Helps, R. M., Rees, N. H. & Hayward, M. A. Sr3Co2O4.33H0.84: an Extended Transition Metal Oxide-Hydride. Inorg. Chem. 49, 11062–11068 (2010).
Tassel, C. et al. Direct Synthesis of Chromium Perovskite Oxyhydride with a High Magnetic-Transition Temperature. Angew. Chem. Int. Ed. 53, 10377–10380 (2014).
Romero, F. D. et al. Strontium Vanadium Oxide-Hydrides: “Square-Planar” Two-Electron Phases. Angew. Chem. Int. Ed. 53, 7556–7559 (2014).
Aleonard, R., Pauthene, R., Rebouill, J. P. & Veyret, C. Interpretation of the Magnetic Properties of the Rare Earth Chromites and the Rare Earth Manganites, J. Appl. Phys. 39, 379 (1968).
Weinberg, I. & Larssen, P. Electron Paramagnetic Resonance and Antiferromagnetism in LaCrO3 . Nature 192, 445–446 (1961).
Togo, A., Oba, F. & Tanaka, I. First-Principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 78, 134106 (2008).
Xiang, H. J., Kan, E. J., Wei, S.-H., Whangbo, M. H. & Gong, X. G. Predicting the Spin-Lattice Order of Frustrated Systems from First Principles. Phys. Rev. B 84, 224429 (2011).
Xiang, H., Lee, C., Koo, H. J., Gong, X. & Whangbo, M. H. Magnetic Properties and Energy-Mapping Analysis. Dalton. Trans. 42, 823–853 (2013).
Mitchell, R. H. & Chakhmouradian, A. R. A New Series of Complex Perovskites La1−xSrxCr1−xTixO3: Structural Characterization. J. Solid State Chem. 144, 81–85 (1999).
Goodenough, J. B., Wickham, D. G. & Croft, W. J. Some Magnetic and Crystallographic Properties of the System Li+xNi++1−2xNi+++xO. J. Phys. Chem. Solids 5, 107–116 (1958).
Kanamori, J. Theory of the Magnetic Properties of Ferrous and Cobaltous Oxides, I. Prog. Theor. Phys. 17, 177–196 (1957).
Kanamori, J. Theory of the Magnetic Properties of Ferrous and Cobaltous Oxides, II. Prog. Theor. Phys. 17, 197–222 (1957).
Anderson, P. W. Antiferromagnetism - Theory of Superexchange Interaction. Phys. Rev. 79, 350–356 (1950).
Wei, Y. et al. The Effect of Hydrogen Ordering on the Electronic and Magnetic Properties of the Strontium Vanadium Oxyhydride. J. Phys. Condens. Matter. 27, 206001 (2015).
Wang, J. et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 299, 1719–1722 (2003).
Tsujimoto, Y. et al. Infinite-layer iron oxide with a square-planar coordination. Nature 450, 1062–1065 (2007).
Xiang, H. J., Wei, S.-H. & Whangbo, M. H. Origin of the Structural and Magnetic Anomalies of the Layered Compound SrFeO2: A Density Functional Investigation. Phys. Rev. Lett. 100, 167207 (2008).
Blöchl, P. E. Projector augmented-wave method, Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comp. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient Iterative Schemes for ab initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An LSDA + U Study. Phys. Rev. B 57, 1505–1509 (1998).
Mostofi, A. A. et al. wannier90: A Tool for Obtaining Maximally-Localised Wannier functions. Comput. Phys. Commun. 178, 685–699 (2008).
Marzari, N., Mostofi, A. A., Yates, J. R., Souza, I. & Vanderbilt, D. Maximally Localized Wannier Functions: Theory and Applications. Rev. Mod. Phys. 84, 1419–1475 (2012).
Ferreira, L. G., Wei, S. H. & Zunger, A. First-principles Calculation of Alloy Phase Diagrams: The Renormalized-Interaction Approach. Phys. Rev. B 40, 3197–3231 (1989).
van de Walle, A., Asta, M. & Ceder, G. The Alloy Theoretic Automated Toolkit: A User Guide. Calphad. 26, 539–553 (2002).
Hukushima, K. & Nemoto, K. Exchange Monte Carlo Method and Application to Spin Glass Simulations. J. Phys. Soc. Jpn. 65, 1604–1608 (1996).
Wang, P. S., Ren, W., Bellaiche, L. & Xiang, H. J. Predicting a Ferrimagnetic Phase of Zn2FeOsO6 with Strong Magnetoelectric Coupling. Phys. Rev. Lett. 114, 147204 (2015).
Acknowledgements
This work was supported by NSFC (11374056), the Special Funds for Major State Basic Research (2012CB921400, 2015CB921700), Program for Professor of Special Appointment (Eastern Scholar), Fok Ying Tung Education Foundation, FANEDD and NCET-10-0351.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, K., Hou, Y., Gong, X. et al. Orbital Delocalization and Enhancement of Magnetic Interactions in Perovskite Oxyhydrides. Sci Rep 6, 19653 (2016). https://doi.org/10.1038/srep19653
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep19653
This article is cited by
-
The role of π-blocking hydride ligands in a pressure-induced insulator-to-metal phase transition in SrVO2H
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.