Abstract
Age at onset (AAO) of bipolar disorders (BD) could be influenced both by a repeat length polymorphism (5HTTLPR) in the promoter region of the serotonin transporter gene (SLC6A4) and exposure to childhood trauma. We assessed 308 euthymic patients with BD for the AAO of their first mood episode and childhood trauma. Patients were genotyped for the 5HTTLPR (long/short variant) and the rs25531. Genotypes were classified on functional significance (LL, LS, SS). A sample of 126 Brazilian euthymic patients with BD was used for replication. In the French sample, the correlation between AAO and trauma score was observed only among ‘SS’ homozygotes (p = 0.002) but not among ‘L’ allele carriers. A history of at least one trauma decreased the AAO only in ‘SS’ homozygotes (p = 0.001). These results remained significant after correction using FDR. Regression models suggested an interaction between emotional neglect and ‘SS’ genotype on the AAO (p = 0.009) and no further interaction with other trauma subtypes. Partial replication was obtained in the Brazilian sample, showing an interaction between emotional abuse and ‘LS’ genotype on the AAO (p = 0.02). In conclusion, an effect of childhood trauma on AAO of BD was observed only in patients who carry a specific stress responsiveness-related SLC6A4 promoter genotype.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is a heterogeneous and complex disorder with a high heritability (close to 60%) and interactions with environmental risk factors (Lichtenstein et al., Lancet, 2009). In order to identify homogeneous subforms of the disorder, candidate symptom such as age at onset (AAO) of the disorder have been suggested1,2. Early AAO has repeatedly been demonstrated to be a valid clinical marker of a subgroup of BD patients with a high familial risk that is coupled to increased comorbid medical and psychiatric disorders and poor prognosis1,2,3. Indeed, early BD onset associates with prototypic clinical characteristics, such as more frequent psychotic symptoms during mood episodes, more manic and mixed episodes and more frequent suicidal behavior. Elevated frequencies of comorbid psychiatric conditions such as panic disorder, conduct disorder, alcohol and/or substance misuses, attention deficit/hyperactivity disorder and of severe medical illnesses such as cardiac, gastro-intestinal and neurological disorders are also observed in patients with an early AAO of BD1,2,4,5. An early onset also associates with a greater severity and a poorer long-term outcome, as evidenced by chronicity and it potential resistance to mood stabilizers, such as lithium salts1,6. Several studies have shown AAO to be a heritable clinical characteristics in families affected by BD1,7,8, although one study failed to replicate these findings9. This intrafamilial resemblance suggests that AAO is determined by common familial factors of genetic and/or environmental origins. However, these factors remain to be clearly identified.
Childhood adverse events may influence the AAO of BD. Two recent reviews of the literature highlighted that the global level of trauma or nonspecific early abuses are associated with an earlier AAO, perhaps especially sexual and physical abuses10,11,12. However, the methodological quality of the reviewed studies has been relatively poor, primarily due to small sample sizes and a lack of standardized assessment of BD diagnosis and childhood trauma. To overcome these methodological difficulties, we recently studied the influence of childhood trauma, using the Childhood Trauma Questionnaire (CTQ), on the clinical expression of BD in a large sample of 587 BD patients from France and Norway13. We found that AAO was lower in patients who had experienced various trauma subtypes, including sexual abuse, emotional abuse, emotional neglect and physical neglect. We also observed a significant dose relationship between childhood abuse and earlier AAO, with AAO being significantly lower in BD patients exposed to more types of abuse. Consequently, childhood trauma(s) are suggested to influence the AAO in BD.
Genetic factors can also influence AAO in BD. Three independent genetic association studies have concluded that a repeat length polymorphism (5HTTLPR) in the promoter region of the SLC6A4 gene (OMIM: 182138) encoding the serotonin transporter, can influence the AAO in BD. An increased presence of the short allele (‘s’) of the 5HTTLPR has been observed in BD patients with an early AAO14. Similarly, in a sample of BD patients of French origin, carriers of the 5HTTLPR ‘ss’ genotype tended to have an earlier AAO15. Finally, a mixture regression analysis also suggested the influence of 5HTTLPR on the AAO in BD16. Overall, these findings suggest that 5HTTLPR could play a role in the modulation of BD AAO. Previous results in a sample of 136 patients with BD suggested that the 5-HTTLPR significantly modulated the relationship between early life stress and age at onset of BD17.
On the basis of such data, this study investigated the interaction between SLC6A4 promoter variants and CTQ (total score and subtypes of abuses and neglects) on the AAO of BD.
Materials and Methods
Samples
The initial sample consisted of euthymic patients with BD type I, II or not otherwise specified (NOS) who were recruited from three psychiatric departments in France (Paris/Créteil, Bordeaux and Nancy). Patient inclusion criteria were: aged over 18 years; having a diagnosis of BD according to DSM-IV criteria18; being Caucasian; and clinically normothymic at the time of inclusion (i.e. having a Montgomery Asberg Depression Rating Scale score19 and a Mania Rating Scale score20 below five as well as no major mood episodes in the last three months). Patients were interviewed using the French version of the Diagnostic Interview for Genetic Studies (DIGS)21, providing lifetime DSM-IV axis I diagnoses18. The AAO of BD was determined retrospectively and was defined as the age at which a patient first met DSM-IV criteria for a major depressive or (hypo)manic episode according to clinical information collected with the DIGS. This sample was a subsample of the one published in 2013 that included 418 patients from France and 169 patients from Norway13. For this study, only patients from France were included and the inclusion was based only on availability of DNA samples. No other exclusion criteria were used (in particular clinical ones). Written informed consent was obtained from all participants. This study was approved by the medical ethics committee of the French << Comité de Protection des Personnes (CPP) >> (IDRCB2008_AO1465_50 VI—Pitié Salpêtrière 118-08) and carried out in accordance with the approved guidelines.
The replication sample consisted of patients with BD (type I, II or NOS) who were recruited from the outpatient Bipolar Disorders Program of Hospital de Clínicas de Porto Alegre (HCPA) in Brazil. Patients were diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV-TR)18. The confirmation of the diagnosis was established by board certified psychiatrists using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)22. The inclusion criteria of the patients were age from 18 to 65 years old, BD diagnosis according to DSM-IV- TR criteria and absence of an acute mood episode during the previous month assessed by means of the Hamilton Rating Scale Score (HDRS)23 and the Young Mania Rating Scale (YMRS)24 <15. The AAO of BD was determined retrospectively and was defined as the age at which a patient first met DSM-IV criteria for a major depressive or (hypo)manic episode according to clinical information collected with the SCID-I. The research ethics committee of HCPA approved the study protocol. All subjects provided written informed consent before their inclusion in the study.
Assessment of childhood affective trauma
Childhood traumatic events were recorded using the CTQ, a 28-item self-report questionnaire25. The CTQ yields a total score and five subscale scores for emotional and physical neglect, as well as emotional, physical and sexual abuses. The CTQ total score was used as a continuous variable assessing trauma severity. Based on the guidelines and cut-off values proposed by Bernstein and Fink26, we used the scores for each subscale to determine whether each kind of trauma was present (mild, moderate or severe) or absent. The French and the Portuguese validated versions were used in this study27,28.
Genotyping
DNA was prepared from peripheral blood leukocytes or B-lymphoblastoïd cell lines by standard procedures. The genotyping of the 5-HTTLPR, rs25531 as well as the triallelic intron 2 VNTR (Stin2) (not used in this study) were performed by triplex polymerase chain reaction (PCR) followed by restriction endonuclease digestion as described by29. Briefly, the amplification reaction was performed using three couples of oligonucleotide primers in a final volume of 20 μl containing 20 ng of genomic DNA and using a 0.5 unit of MyTaq™ DNA Polymerase (Bioline, London, UK). After an initial denaturation at 95 °C for 15 min, the amplification cycles consisted of 35 cycles of denaturation at 95 °C for 30 s, annealing at 65.5 °C for 90 s and elongation at 72 °C for 1 min. Subsequently, 8 μl of amplification products were digested with 5U of HpaII (New England Biolabs, Ipswich, MA, USA), in a 20 μl reaction assay containing 1X NEBuffer 1 and 1X BSA at 37 °C for 1 h. Digested and non-digested PCR products were loaded on 3% agarose gel in 1X TBE. The short ‘s’ and the long ‘l’ alleles (as well as the Stin2.9, Stin2.10 and Stin2.12 alleles not used in this study) were determined from the non-digested PCR products with fragments of 469 bp, 512 bp, 250 bp, 267 bp and 300 bp respectively. The rs25531 A/G polymorphism was determined after HpaII digestion according to the following fragment lengths (LA: 512 bp, LG: 402 + 110 bp, SA: 469 bp and SG: 402 + 67 bp). The more common LA allele is associated with the reported higher basal activity, whereas the less common LG allele has transcriptional activity no greater than the S. Therefore, it is suggested that in tests of association the LG alleles should be analyzed along with the S alleles.
Statistical analyses
The two samples (French and Brazilian) were compared using non-parametric test (Mann-Whitney, Chi-square tests) for descriptive analysis. Since AAO and CTQ scores were assumed not to have a normal distribution, non-parametric tests were used. In this case, Medians and Median Absolute Deviations (MAD) were given. We tested whether the genotype distributions were in Hardy–Weinberg equilibrium.
Linear regression analyses were performed to examine the relationship between AAO, SLC6A4 promoter genotypes and CTQ subtypes as the predictive variables and the interactions between CTQ subtypes and genotypes was used as the confounding factors. The model was AAO transformed ~ Genotypes(LL versus LS versus SS) + CTQ subtype + Genotypes × CTQ subtype. AAO was reciprocal square root transformed in the French sample and square root transformed in the Brazilian sample to fulfill the normality assumption required by the parametric procedure.
We corrected for multiple testing in the French sample, using a FDR (False Discovery Rate) with a p-value = < 0.002 being significant. Statistical analyses were performed with the STATA package (version 12)30.
Results
Description of the French sample
The French sample comprised 308 patients with BD (129 men and 179 women; mean age at interview 43.25 ± 12.6 years old; 229 BD type I, 76 BD type II and 3 BD type NOS). All patients completed the CTQ. Three hundred patients were successfully genotyped for the l/s variant (5HTTLPR) and for the rs25531 (8 DNA failed to be amplified by PCR). The SLC6A4 promoter variants genotypic frequencies were in Hardy-Weinberg equilibrium (p = 0.48).
Influence of the SLC6A4 genotypes on the AAO and on the CTQ total score
The mean AAO of BD was 24.72 ± 9.9 years (median = 22, MAD = 7, range 10–67) and the mean CTQ total score was 42.05 ± 12.9 (median = 39, MAD = 7, range 25–99). AAO and CTQ total score did not differ according to genotypes (see Table 1).
Association between AAO and non-specific trauma according to SLC6A4 genotypes
We first tested for an effect of SLC6A4 genotypes on non-specific level of trauma (i.e. CTQ total score, then a positive history of at least one trauma subtype).
The AAO and the CTQ total score were negatively correlated for the whole sample of patients (rho = − 0.16 p = 0.004). However, this correlation was mainly due to patients carrying the ‘SS’ genotype (rho’SS’ = − 0.32 p = 0.002) and the ‘LS’ genotype (rho’LS’ = − 0.17 p = 0.03) with no correlation observed for those patients carrying the ‘LL’ genotype (rho’LL’ = − 0.02 p = 0.88).
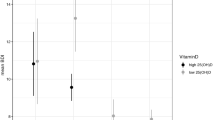
We then considered the effect of the absence/presence of any trauma sub-type (regardless of the subtype considered) on the AAO according to genotypes. AAO decreased in the presence of trauma (one or two and more trauma subtype(s)) in the ‘SS’ subgroup (Wilcoxon rank test: p = 0.001) and to a lesser extend in the ‘LS’ subgroup (p = 0.04) but not in the ‘LL’ subgroup (p = 0.44) (see Fig. 1 for details).
Multivariate regression analyses using CTQ subtypes
Since not all subtypes of trauma are supposed to have equal effects on the AAO with emotional and sexual trauma having possibly a preferential effect13, we tested the following model AAO transformed ~ Genotypes(LLvsLSvsSS) + CTQ subtype + Genotypes × CTQ subtype for each trauma subtype. Transformed AAO (1/sqrt(AAO)) fulfilled the normality assumption (Shapiro-Wilk W = 0.24; p > 0.05).
No significant result was observed when studying physical neglect, physical abuse and emotional abuse. We found an interaction between emotional neglect and ‘SS’ genotype on the AAO (p = 0.009) with a trend for an interaction also between emotional neglect and ‘LS’ genotype on the AAO (p = 0.09) in the same model (see Table 2). We found an association between sexual abuse on the AAO (p = 0.02) with no interaction between this subtype of trauma and genotypes (data not shown).
A power calculation with 5 predictors in the regression models showed that our sample size (n = 300) had a power of 0.76 to identify an effect size of 0.04 (small according to the Cohen’s conventional criteria). A sample size of n = 326 would have been required to reach a power of 0.80 and to identify an effect size of 0.04.
Replication study
The Brazilian sample consisted of 126 patients with BD (23 men and 103 women; mean age at interview 43.03 ± 10.7 years old; 84 BD type I, 40 BD type II and 2 BD type NOS). All patients completed the CTQ with a mean total score of 52.39 ± 20.4. One hundred and twenty two patients were genotyped for the 5HTTLPR l/s variant and for the rs25531 (4 DNA failed to be amplified by PCR). The distribution of genotypes were the following : 30 patients with ‘LL’ genotype, 63 with ‘LS’ genotype and 29 with ‘SS’ genotype. The SLC6A4 promoter variants genotypic frequencies were in Hardy-Weinberg equilibrium (p = 0.82).
The replication sample differed from the initial one for gender distribution (p < 0.001), BD subtypes distribution (p = 0.02) and CTQ total score (p < 0.001); this corresponded to a Brazilian sample consisting of more females, more BD type II and with a higher CTQ total score. The two samples were similar for age at interview (p = 0.99), age at onset (p = 0.59) and genotypes distribution (p = 0.27).
Multivariate regression analyses used transformed AAO (sqrt(AAO)) that fulfilled the normality assumption (Shapiro-Wilk W = 0.98; p > 0.05). No significant result was observed when studying physical neglect, physical abuse, nor sexual abuse. We found an interaction between emotional abuse and ‘LS’ genotype on the AAO (p = 0.02) and a trend for an interaction between emotional neglect and ‘LS’ genotype on the AAO (p = 0.08). No interaction was found with ‘SS’ genotype in both models (see Tables 3 and 4).
A power calculation with 5 predictors in the regression models showed that this replication sample (n = 122) had a power of 0.34 to identify an effect size of 0.04.
Discussion
The identification of factors determining the variability of the AAO in BD is of crucial importance as an early AAO consistently associates with a more severe clinical profile and a poor prognosis1,2,4. The identification of risk factors for an earlier AAO could therefore guide both preventive and more personalized strategies in those at risk of developing BD. Both genetic and environmental determinants are thought relevant to AAO heterogeneity in BD1. In this study, we provided results suggesting an interaction between SLC6A4 promoter variants and emotional trauma on the AAO of BD. In the French sample AAO significantly decreased in the presence of trauma mainly in ‘SS’ carriers, this being significant after correction for multiple testing. Regression analyses suggested (although only nominally significant) that emotional traumatic events might be of special interest when studying environmental risk factors associated with an early occurrence of BD. In the replication sample, the results were partly replicated with some nominal signals in favor an interaction between SLC6A4 promoter variants and emotional trauma on the AAO.
As previously suggested by Benedetti et al.17, this study suggests a gene/environment interaction influencing the AAO of BD and follows several previous findings suggesting that each factor separately decreases the AAO10,11,13,14,15,16. Our data can be placed within gene/environment models of psychiatric conditions, where environmental factors can represent major risk factors for the development of mental disorders, but that their influence is modulated by the presence of susceptibility genetic variants31. Our data highlight a number of processes relevant to the onset and course of BD.
Firstly, our results are consistent with previous pathophysiological data obtained in various experimental models, including knockout mice, stress-reared rhesus macaques and human functional brain imaging32. The serotonin transporter genotype has been shown to be associated with differences in physiological responsiveness to stress conditions in these models, with a relative loss of serotonin transporter gene function being associated with greater vulnerability to environmental stress in all species tested33,34. 5HTTLPR not only regulates serotonergic brain transmission, but carriers of the short allele also display various physiological abnormalities such as increased amygdala reactivity and fear conditioning, as well as alterations in hypothalamo-pituitary-adrenal axis reactivity33. Therefore, short allele carriers might present with a decreased ability to appropriately regulate stress responses, thereby lowering the threshold for the occurrence of a first BD episode.
Second, the mechanisms by which childhood traumatic events decrease the AAO in BD remain unclear. It is likely that this is mediated through the induction of a cascade of neurobiological and neuroendocrine events. Previous studies have suggested that childhood trauma can have long-lasting effects on the catecholamine response to psychological stress35,36 and induce hyper reactivity of the corticoid-releasing factor system37,38,39, as well as altering the structure and function of the medial prefrontal cortex and hippocampus40. Although the effects of such trauma are probably not specific to BD, our data suggests that a history of childhood trauma in BD produces long-term disturbances of neurobiological mechanisms that are required to regulate stress and stress-resilience41, with this then interacting with alterations in genetic regulation of the serotonergic system. Such physiological changes may lower the threshold for the onset of BD, including via sensitization to other environmental triggering factors.
This causal link (i.e. a greater exposure to trauma leading to an earlier AAO) is not the sole interpretation although above mentioned arguments favor it. Other interpretations that consider ontology, parental psychopathology/psychiatric disorders or gene/environment correlations should also be considered. Patients with a ‘SS’ genotype could present with a more pronounced emotional bias toward negative stimuli42 and might thus ‘over-report’ emotional trauma. Second, patients with a ‘SS’ genotype might also present with specific neural defects (such as amygdala over-activation)43 leading them to more sensitivity to negative events and thus, here again, to ‘over-report’ emotional trauma (this hypothesis is probably linked to the emotional attention bias one). In both cases, at-risk genotypes could confer greater attention to and/or more reactivity to emotional trauma. Third, patients with a ‘SS’ genotype might present with other psychiatric disorders (mainly externalized ones, such as attention deficit and hyperactivity disorder, conduct disorder, impulsivity)23,24,44 that are associated with more behavioral problems during childhood/adolescence and thus greater exposure to hard discipline. Although not definitively excluded, these hypotheses favoring a gene/environment correlation are not sustained in our sample since no difference in CTQ scores was observed across genotypes.
The design of this study has several limitations. First, the retrospective assessment of traumatic events during childhood may be influenced by uncontrolled recall bias45. However, previous reviews have suggested that retrospective self-reports concerning childhood abuse are more likely to be biased toward underreporting than exaggeration46,47. Second, the retrospective nature of the AAO assessment also provides a source of potential recall bias, although previous data in this area suggests that this is unlikely48,49. Third, current mood state may lead bipolar patients to under- or over report histories of childhood trauma. We did not adjust CTQ scores for the presence of residual mood symptoms, which we presumed to be very minor, given that we assessed patients during remission periods. As such, potential mood determined biases in early trauma reporting are minimized. Fourth, we did not extensively investigate the genetic structure of SLC6A4, focusing only on the promoter region, nor epigenetic mechanisms. Finally, after correction for multiple testing, we also did not reach significance for in-depth investigations of trauma subtypes. We also were not able to totally replicate our results. This issue was possibly related to sample size and reduced power in the replication sample and incomplete comparability between samples. For example, the number of patients with a ‘SS’ genotype in the replication sample was small (n = 29) and had probably reduce our ability to identify an interaction with this particular genotype. Nevertheless, trends for interaction in the larger ‘LS’ genotype group were suggested as shown in the initial sample. Given these limitations, our results will definitively require replication in independent and larger samples of patients with BD. Indeed, the results of gene/environment interaction studies in psychiatry have been a source of controversy, often being hard to replicate or providing mixed results, as in the case of the interaction between 5HTTLPR, stressful life events and the risk of unipolar depression33,50,51,52,53.
Further research is also required on the genetic and environmental factors that modulate the AAO in BD, which is likely to be multifactorial and determined by many genes with small effect sizes or other variants not measured well by SNP arrays, such as rare alleles or copy number variations54,55,56. Future studies then should investigate other candidate genes that have been suggested to modulate the AAO such as those encoding the apolipoprotein E57, the dopamine receptors DRD1, DRD2 and DRD358,59,60, CACNA1C61, the brain derived neurotrophic factor (possibly in interaction with sexual abuse)62,63, the catechol-O-methyltransferase64, the glucocorticoid and mineralocorticoid receptors65, HTR2A and HTR2C16, Period366, the glycogen synthase kinase 3-beta67,68,69 or SNAP2570,71. Interestingly, we recently demonstrated the interaction between a variant of the Toll-like receptor gene (TLR2) and sexual abuse on the AAO of BD72. Several other environmental factors can also contributed to the modulation of the AAO such as solar insolation, light exposure73,74,75 or exposure to stimulants76, as well as antidepressants77. Among investigated environmental factors that modulate the AAO of BD, the role of early cannabis misuse has been widely proposed4,78,79,80,81,82. The picture is further complicated by the possibility of significant additive effects evident between cannabis abuse and childhood abuse on an earlier AAO in BD81, suggesting the existence of a complex pathway that includes gene-environment modulation and environment/environment interactions. Finally other hypotheses are also relevant to be studied using potential interactions between 5HTTLPR and childhood trauma such as those concerning psychosis or suicidality in BD17,83.
Conclusion
Gene-environment interactions represent a new challenge in psychiatric genetics84 and further studies of these complex interactions on clinical phenotypes are required to increase our understanding of the underlying etiological mechanisms. Such interactions are likely to occur when the effect of exposure to an environmental risk factor on a phenotype depends on the genotype. This study suggests a hypothetical model, in which emotional trauma and SLC6A4 promoter variants interact to influence the AAO in BD. Further investigations in larger samples are required to test this hypothesis in order to help guide preventive strategies among subjects at risk of developing a BD (for example offspring of parents with BD and exposed to trauma).
Additional Information
How to cite this article: Etain, B. et al. Interaction between SLC6A4 promoter variants and childhood trauma on the age at onset of bipolar disorders. Sci. Rep. 5, 16301; doi: 10.1038/srep16301 (2015).
References
Leboyer, M., Henry, C., Paillere-Martinot, M. L. & Bellivier, F. Age at onset in bipolar affective disorders: a review. Bipolar Disord 7, 111–118 (2005).
Geoffroy, P. A. et al. Reconsideration of bipolar disorder as a developmental disorder: Importance of the time of onset. Journal of physiology, Paris 107, 278–285, 10.1016/j.jphysparis.2013.03.006 (2013).
Bellivier, F. et al. Age at onset in bipolar I affective disorder in the USA and Europe. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry 15, 369–376, 10.3109/15622975.2011.639801 (2014).
Etain, B. et al. Clinical expression of bipolar disorder type I as a function of age and polarity at onset: convergent findings in samples from France and the United States. J Clin Psychiatry 73, e561–566, 10.4088/JCP.10m06504 (2012).
Evans-Lacko, S. E., Zeber, J. E., Gonzalez, J. M. & Olvera, R. L. Medical comorbidity among youth diagnosed with bipolar disorder in the United States. J Clin Psychiatry 70, 1461–1466, 10.4088/JCP.08m04871 (2009).
Strober, M. et al. A family study of bipolar I disorder in adolescence. Early onset of symptoms linked to increased familial loading and lithium resistance. J Affect Disord. 15, 255–268. (1988).
O’Mahony, E. et al. Sibling pairs with affective disorders: resemblance of demographic and clinical features. Psychol Med 32, 55–61 (2002).
Lin, P. I. et al. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry 163, 240–246 (2006).
Schulze, T. G., Hedeker, D., Zandi, P., Rietschel, M. & McMahon, F. J. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry 63, 1368–1376 (2006).
Daruy-Filho, L., Brietzke, E., Lafer, B. & Grassi-Oliveira, R. Childhood maltreatment and clinical outcomes of bipolar disorder. Acta Psychiatr Scand 124, 427–434, 10.1111/j.1600-0447.2011.01756.x (2011).
Fisher, H. & Hosang, G. Childhood Maltreatment and Bipolar Disorder: A Critical Review of the Evidence. Mind & Brain, the Journal of Psychiatry, 1–11 (2010).
Maniglio, R. The impact of child sexual abuse on the course of bipolar disorder: a systematic review. Bipolar Disord 15, 341–358, 10.1111/bdi.12050 (2013).
Etain, B. et al. Childhood Trauma Is Associated With Severe Clinical Characteristics of Bipolar Disorders. Journal of Clinical Psychiatry 74, 991–998 (2013).
Ospina-Duque, J. et al. An association study of bipolar mood disorder (type I) with the 5-HTTLPR serotonin transporter polymorphism in a human population isolate from Colombia. Neurosci Lett 292, 199–202 (2000).
Bellivier, F. et al. Serotonin transporter gene polymorphism influences age at onset in patients with bipolar affective disorder. Neurosci Lett 334, 17–20 (2002).
Manchia, M. et al. Mixture regression analysis on age at onset in bipolar disorder patients: investigation of the role of serotonergic genes. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology 20, 663–670, 10.1016/j.euroneuro.2010.04.001 (2010).
Benedetti, F. et al. The serotonin transporter genotype modulates the relationship between early stress and adult suicidality in bipolar disorder. Bipolar Disord 16, 857–866, 10.1111/bdi.12250 (2014).
American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, French Edition: by Masson, Paris. (1994).
Montgomery, S. A. & Asberg, M. A new depression scale designed to be sensitive to change. Br J Psychiatry 134, 382–389 (1979).
Bech, P., Rafaelsen, O. J., Kramp, P. & Bolwig, T. G. The mania rating scale: scale construction and inter-observer agreement. Neuropharmacology 17, 430–431 (1978).
Nurnberger, J. I., Jr. et al. Diagnostic interview for genetic studies. Rationale, unique features and training. NIMH Genetics Initiative. Arch Gen Psychiatry 51, 849–859; discussion 863–844 (1994).
First, M., Sptzer, R., Gibbon, M. & William, J. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P). (New York State Psychiatric Institute., 1995).
Ficks, C. A. & Waldman, I. D. Candidate Genes for Aggression and Antisocial Behavior: A Meta-analysis of Association Studies of the 5HTTLPR and MAOA-uVNTR. Behavior genetics, 10.1007/s10519-014-9661-y (2014).
Gizer, I. R., Ficks, C. & Waldman, I. D. Candidate gene studies of ADHD: a meta-analytic review. Human genetics 126, 51–90, 10.1007/s00439-009-0694-x (2009).
Bernstein, D. P. et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151, 1132–1136 (1994).
Bernstein, D. P. & Fink, L. Childhood Trauma Questionnaire: a retrospective self-report - Manual. (The psychological Corporation—Harcourt Brace and Company. 1998).
Paquette, D., Laporte, L., Bigras, M. & Zoccolillo, M. Validation de la version française du CTQ et prévalence de l’histoire de maltraitance Santé Mentale au Québec 29, 201–220 (2004).
Grassi-Oliveira, R., Stein, L. M. & Pezzi, J. C. [Translation and content validation of the Childhood Trauma Questionnaire into Portuguese language]. Revista de saude publica 40, 249–255, /S0034-89102006000200010 (2006).
Wendland, J. R., Martin, B. J., Kruse, M. R., Lesch, K. P. & Murphy, D. L. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry 11, 224–226, 10.1038/sj.mp.4001789 (2006).
StataCorp . Stata Statistical Software: Release 12. College Station, TX: StataCorp LP (2011).
Uher, R. Gene-environment interactions in severe mental illness. Frontiers in psychiatry 5, 48, 10.3389/fpsyt.2014.00048 (2014).
Hariri, A. R. & Holmes, A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 10, 182–191. Epub 2006 Mar 2010. (2006).
Caspi, A., Hariri, A. R., Holmes, A., Uher, R. & Moffitt, T. E. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry 167, 509–527, 10.1176/appi.ajp.2010.09101452 (2010).
Drabant, E. M. et al. Neural mechanisms underlying 5-HTTLPR-related sensitivity to acute stress. Am J Psychiatry 169, 397–405, 10.1176/appi.ajp.2011.10111699 (2012).
Roy, A. Self-rated childhood emotional neglect and CSF monoamine indices in abstinent cocaine-abusing adults: possible implications for suicidal behavior. Psychiatry Res 112, 69–75 (2002).
Heim, C. & Nemeroff, C. B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49, 1023–1039 (2001).
Braehler, C. et al. Diurnal cortisol in schizophrenia patients with childhood trauma. Schizophr Res 79, 353–354 (2005).
Roy, A. Urinary free cortisol and childhood trauma in cocaine dependent adults. J Psychiatr Res 36, 173–177 (2002).
Heim, C. et al. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety 15, 117–125 (2002).
Bremner, J. D. Neuroimaging of childhood trauma. Semin Clin Neuropsychiatry 7, 104–112 (2002).
Goodman, M., New, A. & Siever, L. Trauma, genes and the neurobiology of personality disorders. Ann N Y Acad Sci 1032, 104–116 (2004).
Pergamin-Hight, L., Bakermans-Kranenburg, M. J., van Ijzendoorn, M. H. & Bar-Haim, Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biol Psychiatry 71, 373–379, 10.1016/j.biopsych.2011.10.030 (2012).
Munafo, M. R., Brown, S. M. & Hariri, A. R. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry 63, 852–857, 10.1016/j.biopsych.2007.08.016 (2008).
Balestri, M., Calati, R., Serretti, A. & De Ronchi, D. Genetic modulation of personality traits: a systematic review of the literature. International clinical psychopharmacology 29, 1–15, 10.1097/YIC.0b013e328364590b (2014).
Hardt, J. & Rutter, M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 45, 260–273. (2004).
Roy, C. A. & Perry, J. C. Instruments for the assessment of childhood trauma in adults. J Nerv Ment Dis. 192, 343–351. (2004).
DiLillo, D. et al. Retrospective assessment of childhood sexual and physical abuse: a comparison of scaled and behaviorally specific approaches. Assessment. 13, 297–312 (2006).
Bellivier, F., Golmard, J. L., Henry, C., Leboyer, M. & Schurhoff, F. Admixture analysis of age at onset in bipolar I affective disorder. Arch Gen Psychiatry 58, 510–512 (2001).
Bellivier, F. et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry 160, 999–1001 (2003).
Caspi, A. et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 (2003).
Karg, K., Burmeister, M., Shedden, K. & Sen, S. The serotonin transporter promoter variant (5-HTTLPR), stress and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry 68, 444–454, 10.1001/archgenpsychiatry.2010.189 (2011).
Munafo, M. R., Durrant, C., Lewis, G. & Flint, J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry 65, 211–219, 10.1016/j.biopsych.2008.06.009 (2009).
Risch, N. et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events and risk of depression: a meta-analysis. JAMA 301, 2462–2471, 10.1001/jama.2009.878 (2009).
Priebe, L. et al. Genome-wide survey implicates the influence of copy number variants (CNVs) in the development of early-onset bipolar disorder. Mol Psychiatry 17, 421–432, 10.1038/mp.2011.8 (2012).
Zhang, D. et al. Singleton deletions throughout the genome increase risk of bipolar disorder. Mol Psychiatry 14, 376–380, 10.1038/mp.2008.144 (2009).
Jamain, S. et al. Common and rare variant analysis in early-onset bipolar disorder vulnerability. PloS one 9, e104326, 10.1371/journal.pone.0104326 (2014).
Bellivier, F. et al. Apolipoprotein E gene polymorphism in early and late onset bipolar patients. Neurosci Lett. 233, 45–48. (1997).
Dmitrzak-Weglarz, M. et al. Dopamine receptor D1 gene -48A/G polymorphism is associated with bipolar illness but not with schizophrenia in a Polish population. Neuropsychobiology 53, 46–50 (2006).
Chiaroni, P. et al. Possible involvement of the dopamine D3 receptor locus in subtypes of bipolar affective disorder. Psychiatr Genet 10, 43–49 (2000).
Squassina, A. et al. Age at onset in bipolar disorder: Investigation of the role of TaqIA polymorphism of DRD2 gene in a Sardinian sample. European psychiatry: the journal of the Association of European Psychiatrists 26, 141–143, 10.1016/j.eurpsy.2010.09.013 (2011).
Zhang, X. et al. Association of genetic variation in CACNA1C with bipolar disorder in Han Chinese. J Affect Disord, 10.1016/j.jad.2013.04.004 (2013).
Min, H. J. et al. Association of the Brain-derived Neurotrophic Factor Gene and Clinical Features of Bipolar Disorder in Korea. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology 10, 163–167, 10.9758/cpn.2012.10.3.163 (2012).
Miller, S. et al. Brain-derived neurotrophic factor val66met genotype and early life stress effects upon bipolar course. J Psychiatr Res 47, 252–258, 10.1016/j.jpsychires.2012.10.015 (2013).
Massat, I. et al. COMT and age at onset in mood disorders: a replication and extension study. Neurosci Lett 498, 218–221, 10.1016/j.neulet.2011.05.012 (2011).
Spijker, A. T. et al. Glucocorticoid and mineralocorticoid receptor polymorphisms and clinical characteristics in bipolar disorder patients. Psychoneuroendocrinology 36, 1460–1469, 10.1016/j.psyneuen.2011.03.020 (2011).
Benedetti, F. et al. A length polymorphism in the circadian clock gene Per3 influences age at onset of bipolar disorder. Neurosci Lett 445, 184–187, 10.1016/j.neulet.2008.09.002 (2008).
Benedetti, F. et al. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett 368, 123–126 (2004).
Lin, Y. F., Huang, M. C. & Liu, H. C. Glycogen synthase kinase 3beta gene polymorphisms may be associated with bipolar I disorder and the therapeutic response to lithium. J Affect Disord 147, 401–406, 10.1016/j.jad.2012.08.025 (2013).
Lee, Y. J. & Kim, Y. K. The impact of glycogen synthase kinase 3beta gene on psychotic mania in bipolar disorder patients. Progress in neuro-psychopharmacology & biological psychiatry 35, 1303–1308, 10.1016/j.pnpbp.2011.04.006 (2011).
Dizier, M. H. et al. Genetic heterogeneity according to age at onset in bipolar disorder: a combined positional cloning and candidate gene approach. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics 159B, 653–659, 10.1002/ajmg.b.32069 (2012).
Etain, B. et al. A SNAP25 promoter variant is associated with early-onset bipolar disorder and a high expression level in brain. Mol Psychiatry 15, 748–755, 10.1038/mp.2008.148 (2010).
Oliveira, J. et al. Combined effect of TLR2 gene polymorphism and early life stress on the age at onset of bipolar disorders. PloS one 10, e0119702, 10.1371/journal.pone.0119702 (2015).
Bauer, M. et al. Impact of sunlight on the age of onset of bipolar disorder. Bipolar Disord 14, 654–663, 10.1111/j.1399-5618.2012.01025.x (2012).
Bauer, M. et al. Influence of light exposure during early life on the age of onset of bipolar disorder. J Psychiatr Res 64, 1–8, 10.1016/j.jpsychires.2015.03.013 (2015).
Bauer, M. et al. Relationship between sunlight and the age of onset of bipolar disorder: an international multisite study. J Affect Disord 167, 104–111, 10.1016/j.jad.2014.05.032 (2014).
DelBello, M. P. et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 3, 53–57 (2001).
Reichart, C. G. & Nolen, W. A. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J Affect Disord 78, 81–84 (2004).
Heffner, J. L., DelBello, M. P., Fleck, D. E., Anthenelli, R. M. & Strakowski, S. M. Cigarette smoking in the early course of bipolar disorder: association with ages-at-onset of alcohol and marijuana use. Bipolar Disord 10, 838–845, 10.1111/j.1399-5618.2008.00630.x (2008).
Lagerberg, T. V. et al. Excessive cannabis use is associated with earlier age at onset in bipolar disorder. European archives of psychiatry and clinical neuroscience 261, 397–405, 10.1007/s00406-011-0188-4 (2011).
Lev-Ran, S., Le Foll, B., McKenzie, K., George, T. P. & Rehm, J. Bipolar disorder and co-occurring cannabis use disorders: Characteristics, co-morbidities and clinical correlates. Psychiatry Res, 10.1016/j.psychres.2012.12.014 (2013).
Aas, M. et al. Additive effects of childhood abuse and cannabis abuse on clinical expressions of bipolar disorders. Psychol Med, 1–10, 10.1017/S0033291713002316 (2013).
Lagerberg, T. V. et al. Indications of a dose-response relationship between cannabis use and age at onset in bipolar disorder. Psychiatry Res 215, 101–104, 10.1016/j.psychres.2013.10.029 (2014).
De Pradier, M., Gorwood, P., Beaufils, B., Ades, J. & Dubertret, C. Influence of the serotonin transporter gene polymorphism, cannabis and childhood sexual abuse on phenotype of bipolar disorder: a preliminary study. European psychiatry: the journal of the Association of European Psychiatrists 25, 323–327, 10.1016/j.eurpsy.2009.10.002 (2010).
Duncan, L. E. & Keller, M. C. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry 168, 1041–1049, 10.1176/appi.ajp.2011.11020191 (2011).
Acknowledgements
We thank patients with BD and controls who agreed to participate in this study. We thank the staff at the inclusion sites in Paris-Créteil (A. Raust and B. Cochet for their active participation in the clinical assessment; E. Abadie for the organization of recruitment and JR Richard for his assistance concerning the ethical procedures), Bordeaux (L. Zanouy) and Nancy (O. Wajsbrot-Elgrabli and RF. Cohen). We are also grateful to the Clinical Investigation Centre (O. Montagne and P. Le Corvoisier) and the Plateforme de Ressources Biologiques (B. Ghaleh) of Mondor Hospital, l’Etablissement Français du Sang of Créteil (J.L. Beaumont and B Mignen), the Cochin Hospital cell library (J. Chelly). This work was supported by INSERM (Institut National de la Santé et de la Recherche Médicale), AP-HP (Assistance Publique des Hôpitaux de Paris), the ITMO Neurosciences, Cognitive Sciences, Neurology and Psychiatry, the Fondation Fondamental (fondation de coopération scientifique) and the Agence Nationale pour la Recherche (ANR NEURO2006, MANAGE_BPAD). This research was also supported the Investissements d’Avenir program managed by the ANR under reference ANR-11-IDEX-0004. The team of M. Leboyer is part of the École des Neurosciences de Paris Ile-de-France network, member of the Bio-Psy Labex and member of the European Network of Bipolar Research Expert Centres (ENBREC).
Author information
Authors and Affiliations
Contributions
B.E., M.L. and F.B. were principal investigators of the study. B.E. and M.L. designed the study, made the analysis and wrote the first draft. A.H. and S.J. performed the genotyping. A.A.D., L.S. and F.K.F. provided the replication sample from Brazil. F. M. provided statistical support. B.E., C.H., S.G. and J.P.K. collected the data for the french sample.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Etain, B., Lajnef, M., Henrion, A. et al. Interaction between SLC6A4 promoter variants and childhood trauma on the age at onset of bipolar disorders. Sci Rep 5, 16301 (2015). https://doi.org/10.1038/srep16301
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16301
This article is cited by
-
Childhood maltreatment, prefrontal-paralimbic gray matter volume, and substance use in young adults and interactions with risk for bipolar disorder
Scientific Reports (2021)
-
Interaction between adverse childhood experiences and polygenic risk in patients with bipolar disorder
Translational Psychiatry (2020)
-
Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression
Molecular Psychiatry (2018)
-
The role of childhood trauma in bipolar disorders
International Journal of Bipolar Disorders (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.