Abstract
Appropriate inhibitory response control is associated with goal-directed behavior. Sleep accelerates the offline consolidation of acquired motor skills that are explicitly predictable; however, the effect of sleep on implicit (unpredictable) motor skills remains controversial. We speculated that a key component of response inhibition skill differentiates between these skill consolidation properties because explicit prediction can minimize the inhibitory efforts in a motor skill. We explored the offline skill learning properties of response inhibition during sleep and wakefulness using auditory Go and Go/Nogo tasks. We attempted to discriminate the possible effects of time elapsed after training (12 or 24 h), post-training sleep/wake state (sleep or wakefulness) and time of day (nighttime or daytime) in 79 healthy human subjects divided into 6 groups that underwent various sleep regimens prior to training and retesting. We found that delayed response inhibition skill improvement was achieved via a simple passage of daytime, regardless of the participants’ alertness level. Our results suggest that sleep-independent neuroplasticity occurs during the daytime and facilitates a delayed learning of response inhibition skill.
Similar content being viewed by others
Introduction
Appropriate inhibitory response control leads to improved goal-directed behavior and reflects an important aspect of psychosocial adaptation to daily life1,2,3. Superior inhibitory response control skills enable us to alter a planned action to an appropriate one in response to an unpredictable change in circumstances. Response inhibition (RI), the ability to inhibit preplanned or ongoing motor action, requires both executive function and subsidiary sensorimotor functions for task switching, control impulsiveness, prepotent response suppression, category shifting and prioritizing actions toward an appropriate goal4,5. RI immaturity or impairment is considered to be involved in the pathology of various mental disorders, such as attention-deficit/hyperactivity disorder6,7 and addictions to gaming, gambling and alcohol8. Because RI skill improvements could result in better psychosocial adaptation6,7,8 and augment psychiatric treatment, developing efficient RI training is an urgent priority.
Stop-signal tasks9,10, including the Go/Nogo task4,11,12,13, are widely used to measure RI in experimental settings. This type of task comprises a major response-accelerating stimulus (e.g., Go or imperative) and a minor response-inhibiting stimulus (e.g., Nogo or stop signal). Although the responses to Go stimuli chiefly reflect a motor acceleration controlled by the motor cortex, the invisible responses to Nogo stimuli, which are closely associated with cognitive inhibition elicited by the central executive system in the prefrontal cortex (PFC)14,15, affect the response to the Go stimuli. Thus, Go/Nogo task performance is able to reflect cognitive inhibition ability better than performance on a simple response task.
Sleep has been shown to have a beneficial role in humans’ offline learning of motor procedural skills16,17,18,19. Walker et al. clearly demonstrated that a night of sleep after skill training results in significant improvements of a motor sequence tapping (MST) skill on a finger-tapping task17, whereas equivalent periods of standby time during wakefulness did not affect participants’ MST skills. Furthermore, a distinctive sleep-dependent MST skill learning profile emerged from the initial practice-dependent improvement during training18,20, suggesting a separate ongoing process of offline MST skill consolidation from the initial acquisition process. In addition to a full night’s sleep, short daytime naps have been suggested to actively contribute to offline MST skill consolidation21,22,23,24. Mednick et al. reported that a brief (60–90 min) daytime nap facilitated an equivalent offline improvement on MST skill24 that was comparable to that following an entire night of sleep16,17, regardless of the time of day of the sleep period.
However, the role of sleep in the offline consolidation of motor procedural skills is still debated. Song, Howard and Howard reported that a probabilistic motor (PM) skill examined with a serial response time task did not improve following sleep in offline PM skill consolidation25. Rather, the PM skill was enhanced to a greater extent after a daytime waking time period had elapsed.
Here, we explore the offline skill consolidation property of RI. A previous study showed that approximately 30-min training immediately improved RI skill, but it did not lead to benefits 4 months after the training26. However, this does not eliminate the possibility of a delayed overnight (offline) improvement in RI skill because the improvement would deteriorate during the 4-month interval without any continuous practice20. It has been shown that working memory (WM) and RI partially share neurocognitive resources such as the left inferior parietal lobule (IPL), right dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), left posterior lateral frontal cortex and supplementary motor area (SMA) to performing flexible and adaptive behavior27,28 and WM skills are consolidated offline29. The offline consolidation of WM skills is associated with increased activity in the middle frontal gyrus and superior and inferior parietal cortices after 5 weeks of WM training30.
To understand the contribution of offline consolidation of RI, we simultaneously considered the effect of sleep state, the crude effects of time of day (nighttime or daytime) of the elapsed time period between training and retest and an interaction between sleep and time of day during offline learning of the RI skill across a day. We utilized an auditory Go/Nogo task that simultaneously measured response time (RT) and hit rate (HR) as indexes of prepotent RI abilities (speed and accuracy) as compared with those in the Go (simple response task) task. The mnemonic property of RI could contribute to psychosocial rehabilitation for various psychiatric disorders.
Results
Sleep habits and experimental sleep control
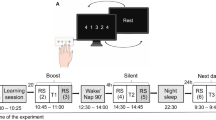
We attempted to discriminate the possible effects of time elapsed after training (12 or 24 h), the post-training sleep/wake state (sleep or wakefulness) and time of day (nighttime or daytime) in 79 healthy human subjects divided into 6 groups that performed various sleep regimens prior to training and retesting (Fig. 1). One-way analyses of variance (ANOVAs) did not show any significant group differences in habitual sleep parameters of sleep onset (F(5, 78) = 0.428, p = 0.733), waking hours (F(5, 78) = 0.524, p = 0.758), or sleep duration (F(5, 78) = 0.624, p = 0.682; Table 1). Participants in groups A, D and E slept during the intersession interval for an average of 7.06 ± 0.21 h (range, 5.11–7.81), 2.49 ± 0.07 h (range, 2.16–2.96) and 7.05 ± 0.22 h (range, 5.12–7.80), respectively.
Experimental schedule.
Time course of the experimental schedule for each group. The intervals between training and retest sessions were 12 h in groups A–D and 24 h in groups E and F. Groups A, B, E and F were trained in the evening (8 p.m.) and groups C and D were trained in the morning (8 a.m.). Groups A and E slept at night as usual, but groups B and F were sleep deprived. Groups C and D performed the experiment in the daytime, whereas group D took a 3-h nap during the training-retest interval (from 1–4 p.m.).
Sleepiness level
A two-way ANOVA revealed a significant main effect of group (F(5,73) = 5.58, p < 0.001) and a group×session interaction (F(5,73) = 7.27, p < 0.001) but showed a non-significant main effect of session (F(5,73) = 0.97, p = 0.489). Subsequent multiple comparisons revealed that participants in groups B (p < 0.001) and F (p < 0.001) felt significantly greater sleepiness during the retest session compared to the training session (Fig. 2), which might have been due to sleep deprivation (SD). However, subjective sleepiness level did not seem to immediately affect Go and Go/Nogo task performance. We did not observe significant correlations between subjective sleepiness and RT in Go (r = −0.199, p = 0.078) and Go/Nogo (r = −0.161, p = 0.154) trainings or Go (r = 0.088, p = 0.436) and Go/Nogo (r = 0.196, p = 0.081) retests. Similarly, we did not note a significant correlation between subjective sleepiness and HR in Go (r = 0.153, p = 0.176) and Go/Nogo (r = −0.179, p = 0.112) trainings or Go (r = 0.088, p = 0.436) and Go/Nogo (r = −149, p = 0.186) retests.
Changes in subjective sleepiness across training-retest intervals.
The subjective sleepiness ratings in groups B and F measured by the visual analog scale (VAS) at the retest session were significantly higher than those at the training session, presumably due to total sleep deprivation during the post-training night. The bars and error bars represent the means and SEMs, respectively. *p < 0.05.
Immediate learning of RI
Lower performances in the Go/Nogo task compared to the Go task suggested appropriate cognitive load by RI on Go/Nogo task performance. The RT on the Go/Nogo training (mean, 379 ms; SEM, 4.09 ms) was significantly slower (t(78) = 11.917, p < 0.0001) than that on the Go training (mean, 316 ms; SEM: 5.51 ms). The HR on the Go/Nogo training (mean, 97.28%; SEM, 0.56%) was significantly lower (t(78) = 4.499, p < 0.0001) than that on the Go training (mean, 99.06%; SEM, 0.29%).
No immediate within-training effects for RT and HR on the Go/Nogo and Go tasks were observed during the training and retest sessions in any of the groups. Two-way ANOVAs showed non-significant main effects of group and training block and non-significant group × training-block interactions for both RT and HR on the Go/Nogo (RT: p > 0.25, HR: p > 0.20) and Go (RT: p > 0.25, HR: p > 0.20) tasks at the training and retest sessions (Fig. 3a–d). In addition, unpaired t-tests showed non-significant differences in training RT (Go: t(77) = 1.426, p = 0.158; Go/Nogo: t(77) = 0.780, p = 0.438) and training HR (Go: t(77) = 0.762, p = 0.449; Go/Nogo: t(77) = −0.051, p = 0.960) caused by training time of day (8 a.m. vs. 8 p.m.). These results suggested that RI skill was not immediately developed by repetitive training and was not affected by the diurnal difference in task execution (8 a.m. or 8 p.m.).
Immediate training profiles on response time (RT) and hit rate (HR) at training and retest sessions.
There were no significant differences among the three blocks of Go and Go/Nogo tasks during the training (a) or retest sessions (b) with regard to RTs or the training (c) and retest sessions (d) with regard to HRs. Open circles and error bars represent the means and SEMs on Go/Nogo performances, respectively and filled circles and error bars represent means and SEMs on Go performances, respectively.
Delayed RI skill learning
A two-way ANOVA for Go/Nogo RT revealed a significant main effect of session (F(1,73) = 14.29; p < 0.001) and a significant group × session interaction (F(5,73) = 4.73; p < 0.001), but it showed a nonsignificant group effect (F(5,73) = 1.842; p = 0.115). Post hoc tests revealed that the Go/Nogo RT in the retest session was significantly faster than that in the training session in groups C (p < 0.001), D (p < 0.001), E (p = 0.049) and F (p = 0.021; Fig. 4a). Go/Nogo HR reached approximately 100% regardless of the session and group. A two-way ANOVA for Go/Nogo HR showed nonsignificant effects of session and group and a nonsignificant group×session interaction (all p > 0.10; Fig. 4b).
No significant differences in the Go/Nogo RT improvement rate were observed among groups C–F. A one-way ANOVA showed no significant main effect of group on the Go/Nogo RT improvement rate (F(3, 52) = 0.755, p = 0.525).
In contrast, Go task performances did not show any significant delayed improvements in any of the groups. A two-way ANOVA for Go RT showed nonsignificant main effects of session and group and a nonsignificant group×session interaction (all p > 0.10; see Table 2). The Go HR also reached approximately 100% regardless of session and group. A two-way ANOVA for Go HR showed nonsignificant main effects of session and group and a nonsignificant group×session interaction (all p > 0.10).
A multiple regression analysis identified daytime (B = 0.496, p < 0.0001) and whole-day (B = 0.370, p = 0.003) denominators of time of day as predictive variables of the Go/Nogo RT improvement rate and did not show any significant differences in the contribution ratio between these denominators (p > 0.05; Fig. 5). However, it excluded all the other variables of sleep state (sleep or wakefulness), training–retest interval (12 or 24 h) and the nighttime denominator of time of day as predictive variables of the Go/Nogo RT improvement rate. In addition, no interactions between predictive variables were observed (all p > 0.10). A high determination coefficient of the model with low collinearity was achieved by these denominators (R2 = 0.196, Fig. 5).
Intersession differences in % improvement in response time (RT) on the Go/Nogo task.
A stepwise multiple linear regression analysis revealed that the daytime (Type II) and whole-day (Type III) denominators of time of day were predictive of the Go/Nogo RT improvement rate. None of the other variables of sleep/wake state, elapsed time duration, or the nighttime (Type I) denominator of time of day from predictive variables of the Go/Nogo RT improvement rate was significant. No interactions between predictive variables were observed.
Discussion
Our results suggest that RI skill in an auditory Go/Nogo task improved during the daytime but not the nighttime offline period and that this occurred regardless of the brain’s sleep/wake state. Here we discuss several confounding factors that could have influenced our results.
The response speed (RT) seemed to be a more sensitive indicator of prepotent RI ability than accuracy (HR), which is in line with the results of previous studies4,11,12,13. Response speed is also more sensitive to the delayed (offline) learning properties of various motor skills compared to accuracy25,31, including sleep-dependent skill consolidation17,18,21. Although immediate training did not result in Go and Go/Nogo performance improvements, a greater delayed improvement of Go/Nogo RT was observed not after an elapsed nighttime period but after an elapsed daytime period regardless of sleep/wake state, elapsed time duration, or alertness. The current results suggested that RI skill exhibited a peculiar diurnal variation in offline learning profiles without sleep/wake state dependency; this is different from other motor skills17,25,32, including those closely engaged in PFC function29. This conclusion was arrived at after ruling out plausible influences of time of day, elapsed time duration between training and retesting and alertness (including possible acute stress of sleep deprivation effects on the Go and Go/Nogo task performances).
Skill learning has been considered to undergo two distinct processes: an immediate (online) within-session process of skill training and a delayed (offline) across-session process of skill learning without ongoing skill training33,34 (see Fig. 6). In the current study, trial repetition within a training session did not improve Go or Go/Nogo performance regardless of the time of day of the initial training session.
Possible confounding factors in immediate (online) task performances and delayed (offline) skill consolidation.
The confounding factors (i–v) could manipulate training–retest Go/Nogo task performance. Although the statistical analyses results demonstrate that the offline improvement in Go/Nogo response time (RT) seems to be directly influenced by the time-of-day effect (ii), the potent time-of-day effect on the online task performance of Go/Nogo RT could make it appear as though there was offline improvement (iv). The effects of sleep/wake state (i), training–retest time duration (iii) on offline RI skill consolidation and the effect of alertness (v) on online RI performances were statistically ruled out.
We did not find any significant time-of-day effects in subjective sleepiness or task performance, but we did observe significant effects of SD on these variables. SD clearly deteriorated subsequent sleepiness but not Go and Go/Nogo performance, although definite trends in sleepiness effects on task performance were observed. Retest Go performance in groups B and F was not deteriorated even though both groups showed greater subjective sleepiness. Previous studies suggested that alertness had a stronger effect on accuracy than on the speed of a simple response task35,36; accuracy was deteriorated by deprivation of a night’s sleep, but speed was not significantly altered35,37,38. However, previous studies used a simple response task that required longer trial repetitions (60 or more) of motor responses; thus, we could not detect a significant influence of SD on Go or Go/Nogo task performance. In other words, alertness has a minimal influence on task performances when a single trial block includes short trial repetitions of motor responses.
A previous study demonstrated an online learning effect of inhibition skill, including Go/Nogo and stop-signal tasks26. The protocol used required a greater amount of RI training repetition (including both the Go/Nogo and the stop signal tasks) than the current study, suggesting that greater trial repetition might unveil a significant training effect of RI skill. Another possible explanation is that we performed an unrecorded adaptive training of the Go and Go/Nogo tasks to confirm PC task operation proficiency, which may have obscured a definitive training effect. Although the non-significant within-training improvement of Go/Nogo RT could mask the meaning of the following across-session improvement in the current study, the across-session improvement of Go/Nogo RT may engage a specific feature of the offline consolidation process during RI skill learning. Because only the intersession intervals of the particular conditions improved the retest Go/Nogo RT regardless of sleep deprivation, it suggests that the improvements of Go/Nogo RT are not simply due to the continuous effect of online training. Instead, resistibility for online training and insidious delayed improvement could be a crucial RI skill property that might complicate the motor skill learning process.
Significant delayed improvements in RT after a 12- or 24-h intersession interval were observed in the groups that contained the elapsed daytime (8 a.m. to 8 p.m.) period regardless of whether those groups had a night’s sleep, a daytime nap, or SD. Moreover, the group-specific delayed improvements in Go/Nogo RT were independent of alertness changes due to SD prior to the retest session. Thus, we considered the elapsed time period in the 12-h time window from 8 a.m. to 8 p.m. to be the most likely source of the offline improvement in RT on the Go/Nogo RI task. In contrast to RI, no significant improvements across intersession interval were observed in HR or RT for the Go task in any of the groups, which is in agreement with previous studies18,21,33,39. Hence, Go task performance robustly reflects the essential motor acceleration component of motor skills and might suggest a ceiling effect of simple motor response skill improvement.
Go/Nogo RT showed significantly delayed improvements across a 12-h intersession interval during the daytime (groups C and D) but not the nighttime (groups A and B). These results highlight two potential characteristics of offline RI skill learning: (1) sleep/wake states are independent of offline RI skill improvement or (2) offline RI skill improvement occurs during a specific time window during the day (8 a.m. to 8 p.m.). However, the remaining problem is that the Go/Nogo RTs in all of the groups retested in the morning (groups A–B) did not improve, whereas they were faster in groups retested in the evening (groups C–F). This suggests that a potential time-of-day effect on Go/Nogo RT in the retest session makes it appear as though Go/Nogo RT improved offline. This is an unlikely scenario because significantly delayed improvements in Go/Nogo RT across the 24-h intersession interval in groups E and F suggest that there is a weak time-of-day effect on training–retest repetition or an influence of alertness on delayed Go/Nogo RT improvement. In addition, there was no detectable time-of-day effect on Go/Nogo RT in the initial training session. Besides, if the improvement in Go/Nogo RT is attributable to a simple diurnal variation in Go/Nogo RT in the retest session, the % improvement in groups A and B should show significant negative changes. As it is, Go/Nogo RT in groups A and B did not show significant intersession differences outside the margin of error. Furthermore, non-significant differences in % improvement of Go/Nogo RT among all of the groups suggested a low probability of an interaction between diurnal variation in the online retest (or training) performances and offline Go/Nogo RT improvement.
Although SD enhanced sleepiness at the retest session for groups B and F, there were no significant differences in delayed improvement rates in Go/Nogo RT compared to groups A and E, respectively. The non-significant intragroup differences in Go/Nogo RT among groups A–D clearly suggested sleep-independent delayed improvement in Go/Nogo RT. The non-significant intergroup differences in % improvement of Go/Nogo RT among groups C–F confirmed that there was no effect of alertness or intersession interval (12 or 24 h) on delayed Go/Nogo RT improvement (Fig. 6).
It should be noted that the difference in offline consolidation between the MST and PM skills could be causally related to the RI skill. The PM skill involves a greater RI component against the accelerating movement with respect to each randomly presented stimulus for a correct response. That is, the MST skill minimizes the inhibition effort by predicting the next movement due to the preplanned motor sequence. In addition, MST skill depends to a large extent on the explicit representation to motor outputs because subjects repeatedly push keys in a known sequence of “explicit awareness.” However, the PM skill was estimated by key press responses to a target that randomly appeared in one of a row of four locations25,40 and did not require explicit knowledge compared with the MST skill. Song et al. inferred from the offline learning properties of PM and MST skills that the sleep-dependent delayed consolidation of MST skill is acquired through its enriched explicit learning process31. Conversely, the implicit (unpredictable) learning process, which dominates PM skill, benefits from the daytime waking period41. Specific motor movements attributed to the primary motor cortex improved because of the offline skill learning process during waking, while motor responses dependent on explicit representation to motor outputs attributed to the hippocampus were improved by the offline skill learning process during sleep31. Furthermore, both explicit representation to motor outputs and implicit contextual information could support predictions regarding the forthcoming motor outputs and facilitate offline motor skill learning during sleep42. In line with this suggestion, RI skill improvement might occur via a sleep-independent offline learning process because the Go/Nogo task is not predictable and requires optimized participant responses.
RI involves inhibition of a prepotent motor response to a stimulus, which requires different neural systems from those involved in pure motor-sequence learning seen in MST skill that are known to show offline improvements during sleep. It has been assumed that RI and WM skills share a similar neural basis in the PFC27 because RI simultaneously requires high-order cognitive functions to optimize behaviors via top-down regulation from the PFC43,44,45. The left frontoparietal network comprises the IFG, pre-SMA, SMA and DLPFC and is actively involved in RI processing15,27,46,47. RI and WM skills both seem to require higher executive PFC function and WM skill improves offline to a greater degree during a sleep period compared to the same period of wakefulness29. However, RI may require different neuroplastic mechanisms. Kuriyama et al. (2008)29 utilized a n-back WM task to investigate a delayed improvement of WM skills and found sleep-dependent improvements. The n-back WM task requires subjects to acquire newly presented stimulus information and simultaneously requires the subject to explicitly maintain a stimulus sequence and respond to questions regarding what stimuli were presented n times before. The WM task dominantly activates the right DLPFC48,49, whereas the Go/Nogo task activates the left IFG and DLPFC27. In addition, WM skills seem to require a greater explicit cognitive component rather than an implicit cognitive component50. These differences between RI and WM might explain the timing difference in the offline skill consolidation process.
Many aspects of human physiology and behavior are under control of the circadian oscillation, although not to the extent seen in wild animals. Human cognition is fundamentally modulated by the circadian processes and memory formation is deeply influenced by sleep-wake homeostasis51. The core molecular circadian oscillator exists as an interlocked transcriptional auto-regulatory feedback loop, including the period (PER) and clock (CLK) genes. Their expression patterns assist rhythmic activation of cellular signaling cascades such as the cyclic adenosine monophosphate (AMP)-mitogen-activated protein kinase (MAPK)-cAMP-responsive element-binding protein (CREB) pathway, which regulates both long-term memory formation and circadian rhythm per se52,53. This pathway is activated during nighttime sleep and is required during the consolidation and reconsolidation of hippocampus-dependent memory (e.g., declarative or explicit memory). Because RI is a nondeclarative and implicit skill, RI skill consolidation may be independent of nighttime cAMP–MAPK–CREB pathway activation.
The study has several potential limitations that made it difficult to clarify the skill consolidation properties of RI. First, our study did not allow disentanglement of the contributions of time-of-day effect and homeostatic effect of sleep deprivation on immediate and delayed improvements. Although a crossover experimental design would verify the interaction between time-of-day and training–retest repetition effects on Go/Nogo task performances, we utilized a between-group design to minimize the cumulative effect of repetitive learning of the RI skill. In addition, circadian effects on retesting are a major concern that was not entirely eliminated by the experimental setting. A possible solution to determine if the observed improvement in groups C–F was due to an offline consolidation effect over daytime or a circadian effect on retesting could be to include an additional control group in which participants are trained in the morning and tested 24 hours later. In this way, the consolidation interval would include a full daytime and retesting would take place in the morning. Second, a consistent but not strictly controlled experimental setting could obscure the possible time-of-day (circadian) and/or alertness effect on Go and Go/Nogo task performances. Previous studies54,55 clearly suggested a circadian variation with higher cognitive task performances and alertness levels in the evening compared to the morning under a constant routine regimen. Some confounders associated with daily life environments could have diminished such sensitive time-of-day influences on task performances in the current study. Third, there was a difference in task difficulty between the Go and Go/Nogo tasks. Lower difficulty in the Go task resulted in a ceiling effect on task performance that could obscure the delayed (offline) learning of the simple response skill in the Go task. In addition, smaller trial repetitions of the Go and Go/Nogo tasks may also obscure immediate (online) training effects25,26, possible time-of-day effects54,55 and possible influences of alertness. However, the consistently higher HR supports the RT findings because of the trade-off between HR and RT. Finally, sleep during the inter-session interval was only evaluated by actigraphic recording. Although actigraphy is a simple and precise method for evaluating sleep duration56, polysomnography is a more accurate and reliable measurement to assess sleep duration and quality. Further research needs to be performed to determine the rigorous circadian effects on offline consolidation of RI skills and the longitudinal effect of RI skill training on human behavior and psychosocial function.
Methods
Participants
Seventy-nine healthy right-handed volunteers (46 females; mean age, 21.4 years; range, 20–26 years) with no previous history of neurological, psychiatric, sleep, or circadian rhythm disorders participated in the study. They were instructed to avoid psychoactive substances such as nicotine, alcohol and caffeine for 24 h before the study period and throughout the experiment. They were asked to maintain a constant sleep-wake schedule from 1 week prior to the end of the experiment and their adherence was confirmed by a sleep assessment questionnaire (Table 1). All participants provided written informed consent prior to their participation. The study protocol was designed in accordance with the Declaration of Helsinki and was approved by the Intramural Research Board of the National Center of Neurology and Psychiatry.
Experimental design
The experiment consisted of a training session and a retest session with a 12- or 24-h intersession interval. Participants were randomly assigned to one of six experimental groups with different intersession interval conditions (groups A–F, Fig. 1). Participants performed an auditory Go/Nogo task in each training and retest session. All test sessions that took place after a night’s sleep or a daytime nap began at least 1 h after awakening to avoid a possible effect of sleep inertia on task performances57,58. Just before the initial training session, each subject performed a brief version of the auditory Go/Nogo task to become familiar with the PC procedure. Throughout the experimental period, all participants stayed in the laboratory and were monitored with an ambulatory wrist activity recorder (Actiwatch-L, Mini-Mitter Co., Inc. Bend, OR) designed to sense movement to distinguish between waking and sleeping states.
To determine whether a subsequent night’s sleep led to any marked improvement in Go and Go/Nogo performance, participants in groups A (n = 14 [8 females; mean age, 21.3 years; range, 20–24 years]) and B (n = 12 [6 females; mean age, 21.2 years; range, 20–25 years]) were trained at 8 p.m. and retested at 8 a.m. the next morning after a 12-h intersession interval. During this interval, group A participants slept in an isolated cabin under dimly lit conditions (<1 lux) from 11 p.m. to 7 a.m. and their sleep was monitored via infrared remote video and an ambulatory wrist activity recorder. Group B participants were deprived of nocturnal sleep and kept awake during the intersession interval in an isolated cabin under normal light conditions (200 lux).
To determine whether a subsequent daytime nap (3 h) led to any marked improvement in the Go and Go/Nogo performances, participants in groups C (n = 13 [6 females; mean age, 21.1 years; range, 20–24 years]) and D (n = 13 [6 females; mean age, 21.2 years; range, 20–24 years]), were trained at 8 a.m. and retested at 8 p.m. after a 12-h intersession interval. Group C participants were awake during the entire intersession interval, while group D participants were forced to nap in an isolated cabin under dimly lit conditions (<1 lux) from 1 p.m. to 4 p.m. During the nap period, they were monitored by infrared remote video and an ambulatory wrist activity recorder.
To confirm whether deprivation of the previous night’s sleep impaired Go and Go/Nogo performance improvements during the subsequent day, participants in group E (n = 13 [6 females; mean age, 21.2 years; range, 20–25 years]) were trained at 8 p.m., subjected to an immediate 8-h sleep period and wakefulness and then retested at 8 p.m. the next day. During the night, group E participants slept in an isolated cabin under dimly lit conditions (<1 lux) and monitored in the same manner as those in groups A and D. Participants in group F (n = 14 [6 females; mean age, 21.2 years; range, 20–25 years]) engaged in the same experimental schedule as group E, but were deprived of nocturnal sleep and kept awake for the 24-h intersession interval.
An auditory Go/Nogo task was employed to elucidate the learning properties of RI skill51,59. An auditory Go task was also used to determine the simple learning properties of the motor response skill as a control. Three trial blocks of the Go/Nogo task (RI task) with three trial blocks of the Go task (simple response task) were run in each training and retest session in a randomized order to minimize the potential bias in task performances caused by inter-block interferences. Each trial block was separated with a 30-s inter-block interval. Each trial block consisted of 20 trials with inter-trial intervals (ITIs: average, 750 ms; range, 500–1000 ms). Participants listened to a sequence of 20 individual 50-ms tone stimuli at 70 decibels in a trial block via headphones. The sequence consisted of 15 low-pitched tone stimuli (75%) with 1000-Hz frequency and 5 high-pitched tone stimuli (25%) with 1200-Hz frequency. Participants had to respond to all tones by pressing a button as quickly and accurately as possible in the Go trial blocks. Participants had to respond to low-pitched tones by pressing a button as quickly and accurately as possible but were asked to withhold a response to the high-pitched tones in the Go/Nogo trial blocks. Thus, 20 and 15 responses were obtained in a trial block of the Go and Go/Nogo tasks, respectively. RT and HR were calculated in each block and session. Responses made after 500 ms from the offset of tone stimuli were considered faults.
At the beginning of each session, the subjective sleepiness of each participant was measured with a visual analog scale (VAS). The VAS consisted of a 100-mm-long horizontal line labeled “extremely alert” on the left and “extremely sleepy” on the right. Participants drew a vertical mark on the line at a point corresponding to their level of sleepiness. Higher scores indicated greater subjective sleepiness.
Statistics
ANOVAs and multiple comparisons were used to examine task performance (RT and HR) and sleepiness level. Two-way, between-group (six groups)×within-trial block (three trial blocks), ANOVAs were conducted for Go and Go/Nogo performances during the online training session. Two-way, between-group (six groups)×within-session (two sessions), ANOVAs were conducted for Go and Go/Nogo performances and subjective sleepiness levels across the training–retest sessions. A one-way between-group ANOVA was conducted to examine group differences in offline improvement rates (% improvements) in Go and Go/Nogo performances. Pearson’s correlation coefficients were calculated to examine the influence of alertness (subjective sleepiness) on Go and Go/Nogo performances. Unpaired t-tests were used to examine the influence of time of day (8 a.m. or 8 p.m.) on task execution on online Go and Go/Nogo performances. Stepwise multiple linear regression analyses were performed to identify independent predictors of offline improvements in Go/Nogo performances, such as sleep state (sleep or wakefulness), time of day (nighttime, daytime, or whole-day) and training–retest interval (24-h or 12-h) using a dummy variable adjustment method. A variance inflation factor (VIF)≥10 was regarded as significantly serious multi-collinearity. All tests were two-tailed. The results are shown as the mean and standard error of the mean (SEM). A p-value of 0.05 was considered statistically significant, but a corresponding Bonferroni-adjusted p value for each statistical level was set for each post-hoc test. SPSS 16.0 J for Windows (SPSS, Inc., Chicago, IL) was used for the statistical analyses.
Additional Information
How to cite this article: Honma, M. et al. Sleep-independent offline consolidation of response inhibition during the daytime post-training period. Sci. Rep. 5, 10362; doi: 10.1038/srep10362 (2015).
References
Blair, C. & Diamond, A. Biological processes in prevention and intervention: the promotion of self-regulation as a means of preventing school failure. Dev. Psychopathol. 20, 899–911 (2008).
Rushworth, M. F. Intention, choice and the medial frontal cortex. Ann. N.Y. Acad. Sci. 1124, 181–207 (2008).
Filevich, E., Kühn, S. & Haggard, P. Intentional inhibition in human action: the power of ‘no’. Neurosci. Biobehav. Rev. 36, 1107–1118 (2012).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177 (2004).
Friedman, N. P. & Miyake, A. The relations among inhibition and interference control functions: A latent-variable analysis. J. Exp. Psychol. Gen. 133, 101–135 (2004).
Alderson, R. M., Rapport, M. D. & Kofler, M. J. Attention-deficit/hyperactivity disorder and behavioral inhibition: a meta-analytic review of the stop-signal paradigm. J. Abnorm. Child Psychol. 35, 745–758 (2007).
Groman, S. M., James, A. S. & Jentsch, J. D. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 33, 690–698 (2009).
Goudriaan, A. E., Oosterlaan, J., de Beurs, E. & van den Brink, W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction 101, 534–547 (2006).
Stahl, J. & Gibbons, H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: an electrophysiological investigation using a stop-signal task. Clin. Neurophysiol. 118, 581–596 (2007).
Proulx, T., Inzlicht, M. & Harmon-Jones, E. Understanding all inconsistency compensation as a palliative response to violated expectations. Trends Cogn. Sci. 16, 285–291 (2012).
Kramer, A. F., Humphrey, D. G., Larish, J. F., Logan, G. D. & Strayer, D. L. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol. Aging 9, 491–512 (1994).
Eimer, M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol. Psychol. 35, 123–138 (1993).
Hester, R., Fassbender, C. & Garavan, H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb. Cortex. 14, 986–994 (2004).
Menon, V., Adleman, N. E., White, C. D., Glover, G. H. & Reiss, A. L. Error-related brain activation during a Go/NoGo response inhibition task. Hum. Brain Mapp. 12, 131–143 (2001).
Swick, D., Ashley, V. & Turken, A. U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56, 1655–1665 (2011).
Fischer, S., Hallschmid, M., Elsner, A. L. & Born, J. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. U.S.A. 99, 11987–11991 (2002).
Walker, M. P., Brakefield, T., Morgan, A., Hobson, J. A. & Stickgold, R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35, 205–211 (2002).
Kuriyama, K., Stickgold, R. & Walker, M. P. Sleep-dependent learning and motor-skill complexity. Learn. Mem. 11, 705–713 (2004).
Arzi, A. et al. Humans can learn new information during sleep. Nat. Neurosci. 15, 1460–1465 (2012).
Walker, M. P., Brakefield, T., Hobson, J. A. & Stickgold, R. Dissociable stages of human memory consolidation and reconsolidation. Nature 425, 616–620 (2003).
Stickgold, R. Sleep-dependent memory consolidation. Nature 437, 1272–1278 (2005).
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010).
Korman, M. et al. Daytime sleep condenses the time course of motor memory consolidation. Nat. Neurosci. 10, 1206–1213 (2007).
Mednick, S. C., Cai, D. J., Kanady, J. & Drummond, S. P. Comparing the benefits of caffeine, naps and placebo on verbal, motor and perceptual memory. Behav. Brain Res. 193, 79–86 (2008).
Song, S., Howard Jr., J. H. & Howard, D. V. Implicit probabilistic sequence learning is independent of explicit awareness. Learn. Mem. 14, 167–176 (2007).
Enge, S. et al. No evidence for true training and transfer effects after inhibitory control training in young healthy adults. J. Exp. Psychol. Learn. Mem. Cogn. 40, 987–1001 (2014).
Criaud, M. & Boulinguez, P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI?A meta-analysis and critical review. Neurosci. Biobehav. Rev. 37, 11–23 (2013).
Swick, D., Ashley, V. & Turken, A. U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 9, 102 (2008).
Kuriyama, K., Mishima, K., Suzuki, H., Aritake, S. & Uchiyama, M. Sleep accelerates the improvement in working memory performance. J. Neurosci. 28, 10145–10150 (2008).
Olesen, P. J., Westerberg, H. & Klingberg, T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 7, 75–79 (2004).
Song, S., Howard, J. H. & Howard, D. V. Sleep does not benefit probabilistic motor sequence learning. J. Neurosci. 27, 12475–12483 (2007).
Debas, K. et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl. Acad. Sci. U.S.A. 107, 17839–17844 (2010).
Karni, A., Tanne, D., Rubenstein, B. S., Askenasy, J. J. & Sagi, D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265, 679–682 (1994).
Robertson, E. M., Pascual-Leone, A. & Press, D. Z. Awareness modifies the skill-learning benefits of sleep. Curr. Biol. 14, 208–212 (2004).
Basner, M. & Dinges, D. F. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 34, 581–591 (2011).
Miccoli, L., Versace, F., Koterle, S. & Cavallero, C. Comparing sleep-loss sleepiness and sleep inertia: lapses make the difference. Chronobiol. Int. 25, 725–744 (2008).
Adam, M., Rétey, J. V., Khatami, R. & Landolt, H. P. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep 29, 55–57 (2006).
Lee, I. S., Bardwell, W. A., Ancoli-Israel, S. & Dimsdale, J. E. Number of lapses during the psychomotor vigilance task as an objective measure of fatigue. J. Clin. Sleep Med. 6, 163–168 (2010).
Gaab, N., Paetzold, M., Becker, M., Walker, M. P. & Schlaug, G. The influence of sleep on auditory learning: a behavioral study. Neuroreport 15, 731–734 (2004).
Nissen, M. J. & Bullemer, P. Attentional requirements of learning: Evidence from performance measures. Cogn. Psychol. 19, 1–32 (1987).
Fischer, S., Drosopoulos, S., Tsen, J. & Born, J. Implicit learning -- explicit knowing: a role for sleep in memory system interaction. J. Cogn. Neurosci. 18, 311–319 (2006).
Spencer, R. M., Sunm, M. & Ivry, R. B. Sleep-dependent consolidation of contextual learning. Curr. Biol. 16, 1001–1005 (2006).
Baddeley, A. D. & Hitch, G. J. Working Memory in The psychology of learning and motivation, Vol. 8 (ed Bower, G. A. ), 47–89 (Academic Press, 1974).
Smith, E. E. & Jonides, J. Storage and executive processes in the frontal lobes. Science 283, 1657–1661 (1999).
Miller, E. K. & Cohen, J. D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001).
Zhang, S. & Li, C. S. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum. Brain Mapp. 33, 89–104 (2012).
Hirose, S. et al. Efficiency of go/no-go task performance implemented in the left hemisphere. J. Neurosci. 32, 9059–9065 (2012).
Taylor, S. F. et al. A functional neuroimaging study of motivation and executive function. Neuroimage 21, 1045–1054 (2004).
Crone, E. A., Wendelken, C., Donohue, S., van Leijenhorst, L. & Bunge, S. A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U.S.A. 103, 9315–9320 (2006).
Manginelli, A. A., Baumgartner, F. & Pollmann, S. Dorsal and ventral working memory-related brain areas support distinct processes in contextual cueing. Neuroimage 67, 363–374 (2013).
Eigsti, I. M. et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol. Sci. 17, 478–484 (2006).
Gerstner, J. R. et al. Cycling behavior and memory formation. J. Neurosci. 29, 12824–12830 (2009).
Gerstner, J. R. & Yin, J. C. Circadian rhythms and memory formation. Nat. Rev. Neurosci. 11, 577–588 (2010).
Wyatt, J. K., Ritz-De Cecco, A., Czeisler, C. A. & Dijk, D. J. Circadian temperature and melatonin rhythms, sleep and neurobehavioral function in humans living on a 20-h day. Am. J. Physiol. 277, R1152–1163 (1999).
Dijk, D. J., Duffy, J. F. & Czeisler, C. A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 17, 285–311 (2000).
Ancoli-Israel, S. et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26, 342–392 (2003).
Silva, E. J. & Duffy, J. F. Sleep inertia varies with circadian phase and sleep stage in older adults. Behav. Neurosci. 122, 928–935 (2008).
Scheer, F. A., Shea, T. J., Hilton, M. F. & Shea, S. A. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J. Biol. Rhythms 23, 353–361 (2008).
Dimitrov, M. et al. Inhibitory attentional control in patients with frontal lobe damage. Brain Cogn. 52, 258–270 (2003).
Acknowledgements
We thank Sayori Koyama, Miyuki Shimazaki and Mikiko Kimura for assistance with the experiments. This work was supported by Grants-in-Aid for Scientific research (#24650142, #21790235 and #25670122) from the Japan Science and Technology (JST) Corporation, the Japan Foundation for Neuroscience and Mental Health, the Takeda Science Foundation and the SENSHIN Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
K.K. designed the study. M.H., T.Y., H.I. and K.K. performed the experiments. M.H. and K.K. carried out statistical analyses and wrote the main text. All authors contributed to the final version of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Honma, M., Yoshiike, T., Ikeda, H. et al. Sleep-independent offline consolidation of response inhibition during the daytime post-training period. Sci Rep 5, 10362 (2015). https://doi.org/10.1038/srep10362
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10362
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.