Abstract
Nb2O5 nanosheets are successfully synthesized through a facile hydrothermal reaction and followed heating treatment in air. The structural characterization reveals that the thickness of these sheets is around 50 nm and the length of sheets is 500 ~ 800 nm. Such a unique two dimensional structure enables the nanosheet electrode with superior performance during the charge-discharge process, such as high specific capacity (~184 mAh·g−1) and rate capability. Even at a current density of 1 A·g−1, the nanosheet electrode still exhibits a specific capacity of ~90 mAh·g−1. These results suggest the Nb2O5 nanosheet is a promising candidate for high-rate lithium ion storage applications.
Similar content being viewed by others
Introduction
Lithium ion batteries are commonly used for consumer electronics, portable electric devices, electric vehicles and other applications1,2,3,4,5. However, for high power density applications such as in electric vehicles, it is hindered by the achievement of high-rate capability of electrode materials. Recently, transition metal oxides have been extensively explored as anode replacing graphite due to their higher theoretical capacity and high packing densities, which lead to high volumetric energy densities in devices6,7,8,9,10.
Among the different transition metal oxide as anode materials, lithium titanate (Li4Ti5O12) and niobium oxide (Nb2O5) have received the most study because of the considerable safety advantage that their redox potentials match to the LUMO of the organic liquid-carbonate electrolyte11,12,13. Compared with Li4Ti5O12 with a specific capacity of 140 mAh·g−1, Nb2O5 exhibits a higher capacity (~200 mAh·g−1)11,12. Furthermore, Augustyn and Dunn et al found that the crystal structure of orthorhombic Nb2O5 permits exceptionally rapid ionic transport since the mostly empty octahedral sites between (001) planes provide natural tunnels for lithium ion transport throughout the a–b plane14,15, which makes Nb2O5 a promising anode material. However, its intrinsic poor electric conductivity (σ ~ 3 × 10−6 S·cm−1) and the capacity decay resulted from pulverization during charge-discharge process limit its practical application in lithium ion batteries and thus it is still challenging to develop efficient but simple ways to enhance the utilization of electroactive Nb2O515,16,17,18,19.
Building nanostructures with desirable morphology and size is of great importance for addressing this issue15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. For instance, Dunn et al developed Nb2O5 mesoporous films through a simple solution process, which exhibited high-rate lithium insertion capability16. Wang and Lu et al reported high performance supercapacitors based on nanocomposites of Nb2O5 nanocrystals and carbon nanotubes17. Nb2O5-carbon core-shell nanocomposites were fabricated by Li and Ma et al, which exhibited high specific capacity and rate capability18. Nb2O5 nanobelts and hollow nanospheres were also been developed with large capacity and high rate capability19,20.
Among these nanostructures, two dimensional structures with nano size in thickness and micro size in length have been considered to be the appropriate morphology for energy storage22,23,24,25. Generally, a nano-sized thickness has short ion diffusion path and large surface area; the micro-sized length can lower the internal resistance and facilitate the electron transfer rate as compared with the noncontinuous oxide framework composed of nanoparticles22,23,24,25. These merits encourage us to investigate Nb2O5 electrode materials with two dimensional structures.
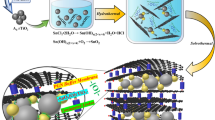
In this work, Nb2O5 nanosheets were developed by a two-step hydrothermal reaction and subsequent calcination process. The two dimensional sheet-like structure was composed of thin thickness (~50 nm) and long length (~800 nm), fitting the desirable structure principles as mentioned above. As expected, the nanosheet electrodes exhibited superior capacity (~184 mAh·g−1), much higher than commercial Nb2O5 particles (~135 mAh·g−1). Additionally, the samples performed well at high current density (~130 mAh·g−1 at 0.4 A·g−1 and ~90 mAh·g−1 at 1 A·g−1), indicating its high rate capability. These results demonstrate that our samples are promising for the future practical application in lithium ion storage.
Results
Synthesis of intermediate Nb3O7F nanosheets
As shown in Fig. 1a, XRD pattern of the as-obtained powders from hydrothermal process can be indexed as an orthorhombic Nb3O7F structure (JCPDS Card No. 74-2363). Fig. 1b shows the morphology of the as-prepared Nb3O7F, displaying two dimensional sheet-like structure. A TEM image of Nb3O7F nanosheets after ultrasound treatment is shown in Fig. 1c. It can be observed that these Nb3O7F nanosheets are rectangular shape. The nanosheets are almost transparent, suggesting the sheet is very thin. The size of these sheets is around 500 ~ 800 nm. The HRTEM image (Fig. 1d) and SAED pattern (top inset in Fig. 1d) reveal a good single crystalline nature of the Nb3O7F nanosheets. The lattice fringes show an interplanar spacing of ca. 0.38 nm and 0.39 nm (Fig. 1d), corresponding to the (010) and (001) planes of the orthorhombic Nb3O7F, respectively.
We investigated the effect of different hydrothermal conditions on the resultant Nb3O7F crystal structure and morphologies. The reaction time has been found playing a crucial role in controlling the nucleation and growth of crystallites during the hydrothermal system. As shown in Fig. 2, XRD patterns of the solids samples prepared at 160°C with different reaction time indicates pronounced changes in crystal structure during the reaction. XRD patterns of the sample obtained in 3 h are different from that of raw materials Nb, suggesting a new crystal phase forms at the expense of Nb in a short period. With prolonging the reaction time to 6 h, a new diffraction peak appears at 2θ = 22.5°, which can be indexed as Nb3O7F. Increasing the reaction time to 8 h, it can be found that the diffraction peak at 2θ = 36.6° almost disappear, indicating the crystal phase formed in 3 h degrades readily in the subsequent reaction process. All diffraction peaks of the sample prepared with 12 h are indexed to Nb3O7F (JCPDS card No. 74-2363). Further prolonging the reaction time to 24 h or 48 h, the XRD patterns are similar, demonstrating the as-obtained samples are pure Nb3O7F with the reaction time at and longer than 12 h.
The morphology evolution of Nb3O7F with increasing reaction times was also studied. As shown in Fig. 3a, the samples formed in 3 h are particles. With the reaction time prolonging to 6 h, one dimensional rod-like structure can be observed. The diameter of these rods is around 30 nm (Fig. 3b). Further study indicates that these nanorods appear as a metastable intermediate. For instance, XRD results clearly demonstrate that some new diffraction peaks of Nb3O7F appear after 8 h of reaction. In addition, this has also been confirmed by SEM observation. With increasing the reaction time to 8 h, some sheets are formed besides those nanorods. The thickness of sheets is around 20 nm and the size is 600 ~ 800 nm. Fig. 3d reveals the presence of a large number of sheets, which indicates that the purity of sheets in the product is increased by prolonging the reaction time. XRD results confirm that the products obtained after reacting 12 h are pure Nb3O7F. The samples prepared in 24 h are well-crystallized sheets, as shown in Fig. 3e. The thickness of these sheets is around 50 nm, much thicker than that of sheets obtained in 8 h. However, it can be found that most of sheets crack into small pieces with further prolonging the reaction time to 48 h, indicating too long reaction time may damage the perfect sheet-like structure. Therefore, it can be concluded that 24 h is the optimized reaction condition for preparing Nb3O7F nanosheets.
Synthesis of Nb2O5 nanosheets
Ultrathin Nb2O5 nanosheets could be developed by an in-situ heat treatment of Nb3O7F nanosheets prepared from optimized reaction condition in air. Fig. 4a shows the XRD pattern of Nb3O7F heated at 550°C for 1 h. All diffraction peaks are indexed to orthorhombic Nb2O5 (JCPDS card No. 30-0873). From SEM image in Fig. 4b, it can be observed that the sheet-like structure was kept very well after heating treatment. This is also confirmed by the TEM observation, as shown in Fig. 4c. A typical HRTEM image (Fig. 4d) discloses the lattice fringes with a spacing of 0.39 nm, in a good agreement with the spacing of the (001) planes of Nb2O5. The results indicate the (001) planes are perpendicular to nanosheets, which may provide natural tunnels for lithium ion transport throughout the a–b plane15 and thus favour fast intercalation/de-intercalation reaction.
Electrochemical performance of Nb2O5 nanosheets
High-valent Nb2O5 is well known to act as electrode material for lithium intercalation (xLi + xe− + Nb2O5 → LixNb2O5). The amount of lithium insertion in LixNb2O5 varies between x = 1.6 to a maximum capacity of 200 mAh·g−1 (x = 2). Here, the electrochemical properties of lithium ions intercalation (or deintercalation) into (or from) Nb2O5 nanosheets are characterized. As shown in Fig. 5a, the CV curves of Nb2O5 at a scan speed of 0.5 mV/s in a potential window of 2.5 to 1.0 V (vs. Li+/Li) present symmetric cathodic and anodic peaks, indicating a reversible lithium intercalation and de-intercalation process. Representative voltage profiles at second cycle is shown in Fig. 5b. The curve shows highly reversible capacities.
Electrochemical properties of the Nb2O5 nanosheet electrodes.
(a) CV curves of Nb2O5 nanosheet electrodes; (b) second galvanostatic discharge/charge profiles under 1 C; (c) cycling performance and Coulombic efficiency of the Nb2O5 nanosheet and nanoparticle electrodes under 1 C; (d) rate performance of the Nb2O5 nanosheet electrodes; (e) cycling performance and Coulombic efficiency of the Nb2O5 nanosheet electrodes under 5 C.
Fig. 5c compares the charge storage and Coulombic efficiencies of the electrodes from Nb2O5 nanosheets and commercial Nb2O5 particles at 0.2 A·g−1 (1 C). The initial discharge capacity of this nanosheet electrode is 184 mAh·g−1, which almost reaches the theoretical capacity (200 mAh·g−1), while the initial discharge capacity of commercial Nb2O5 particles electrode is only 135 mAh·g−1, much lower than that of nanosheet electrode. The capacity can keep 117 mAh·g−1 and 83 mAh·g−1 after 100 cycles for the nanosheet and particle electrodes, respectively. Kodama et al found that the continuous variation in the valence state from Nb5+ to Nb4+ takes place in the discharge reaction, as xLi + xe− + Nb2O5 → LixNb2O5 (x = 0–2)21. This structural variation during the Li intercalation of the two phases could induce strain, which may influence the structure integrity of nanosheets. The structure change of nanosheets could deteriorate the contact between active materials and conducting additives and thus lead to the capacity fading. The similar phenomenon was also observed in nanobelt electrodes19. It should be noted that the Coulombic efficiencies of nearly 100% are achieved for both nanosheet and particle electrodes in this work.
To investigate the rate-capability, the electrode was charged and discharged at 1C to 5C. Fig. 5d shows the rate performance of the nanosheet electrodes. As expected, the specific capacity decreases with the increase of current densities. But the good capacity can be switched back to 2C and 1C again. Furthermore, the nanosheet electrode still exhibits a specific capacity of ~90 mAh·g−1 at 5C, indicates the fast reaction kinetics in the electrodes. Fig. 5e shows the cycling performance of the nanosheet electrode at 5C for 200 cycles. It can be found that the specific capacity is around 90 mAh·g−1 at the first cycle at 5C and around 70 mAh·g−1 after 200 cycles, with Coulombic efficiency stabilized at 100%. The capacity decay rate is as low as 0.11%, which is considered very good for metal oxide nanostructures based electrode materials20. The results well demonstrate that Nb2O5 nanosheets are promising anode materials for high-rate lithium ion storage applications.
Discussion
Growth mechanism of intermediate Nb3O7F nanosheets
Based on XRD and SEM results (in Fig. 2 and Fig. 3), a possible Nb3O7F nanosheets growth process is presented here. Firstly, Nb powders are slowly etched to H2NbF7 by HF and then H2NbF7 is hydrolyzed into Nb3O7F. With increasing the hydrothermal reaction time, the Nb3O7F concentration in reaction solution was further enhanced, which resulted in the formation of Nb3O7F nanosheets. The chemical reaction process in this hydrothermal system is similar to the results reported by other researchers32.
Improving the electrochemical performance of Nb2O5 electrodes through constructing 2D microstructures
It is obvious that the specific capacity of Nb2O5 nanosheet electrode is much higher than that of Nb2O5 particles, as shown in Fig. 5c. The improved electrochemical performance could be related to the following structural features. First, the micro-sized length (~1 μm) provides a continued pathway for charge transfer, which makes the redox reaction rate faster. Second, the thin thickness (~50 nm) increases the specific surface area, which results in the improvement of the contact area between electrode and electrolyte and facilitated the diffusion of electrolyte into the material, leading to more efficient utilization of the active materials. Third, the (001) planes perpendicular to nanosheets allow degenerate pathways with low energy barriers for ion transport, which may also contribute to the improvement of cycling performance15. As shown in Fig. 5e, it can be found that the capacitance retention of 200th cycle is 78% compared to the first cycle at 5C. The nanosheets exhibit superior cycling stability than those nanobelts reported by Wei et al, which retains only 72% of the initial discharge capacity after 50 charge/discharge cycles at 0.5C19. The superior performance of nanosheets can be attributed to the unique morphology that these sheets not only provide short Li-ion transport length but also accommodate the volume variation. In addition, Sasidharan et al reported that their hollow nanospheres also exhibit excellent cycle stability20. The capacity retention of 250th cycle is 90% compared to the first cycle20. They attribute this excellent cycle performance to the thin-shell of hollow spheres with 6 nm favoring fast intercalation/deintercalation reaction and void space effectively buffering against the local volume changes during repeated charge/discharge processes20. As a consequence, the performance of nanosheets could be further improved if the thickness could be reduced in future.
In summary, we have successfully synthesized Nb2O5 nanosheets from its precursor Nb3O7F nanosheets. The nanosheet electrode delivers a superior electrochemical performance with an initial discharge capacity of 184 mAh·g−1 at 0.2 A·g−1 current density. Cycling measurement suggest Nb2O5 nanosheet electrodes show a high reversible charge/discharge capacity, high rate-capability and excellent cycling stability, making this material a good candidate as an electrode for high-rate electrochemical energy storage applications.
Methods
Materials Synthesis
Synthesis of Nb2O5 nanosheets was performed using the metal Nb powder as the starting materials. In a typical synthesis, 0.15 g Nb metal powder and 0.3 mL HF were added into 30 mL distilled water, then the mixture solution was transferred into a 50 mL Teflon-lined autoclave and kept it in oven at 160°C for 3 ~ 48 h. The as-prepared precursor was then annealed at 550°C for 1 h in air to obtain Nb2O5 nanosheets.
Materials Characterization
The morphologies of precursor and annealed samples were investigated using a field emission scanning electron microscope (FE-SEM, Hitachi S4800) and a field emission transmission electron microscopy (FE-TEM, FEI, Tecnai G2 F20 S-Twin). X-ray diffraction (XRD, Bruker AXS, D8 Advance) was used for crystal structure characterization.
Electrochemical Measurements
The as-obtained Nb2O5 nanosheets were mixed with super P and polyvinylidene fluoride (PVDF, Mw = 560 K) in a weight ratio of 80 (active materials):10 (super P):10 (binder) and 1-Methyl-2-pyrrolidinon (NMP) was added to form a homogenous slurry. The slurry was cast onto aluminum current collector using the doctor blade technique. The cast electrode was dried in a vacuum oven at 100°C for 5 h and punched into 15 mm circular discs. CR2016 coin cells were assembled in an Ar-filled glove box with a lithium metal foil as counter electrode and a porous polypropylene separator (2400, Celgard). The electrolyte consisted of a solution of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC)/diethyl carbonate (DEC) (1:1:1 by volume).
References
Dunn, B., Liu, P. & Meng, S. Nanoscience and Nanotechnology in Next Generation Lithium Batteries. Nanotech. 24, 420201 (2013).
Stevenson, K., Ozolins, V. & Dunn, B. Electrochemical Energy Storage. Acc. Chem. Res. 46, 1051–1052 (2013).
Ji, L. et al. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 133, 18522–18525 (2011).
Song, M., Zhang, Y. & Cairns, E. J. A Long-Life, High-Rate Lithium/Sulfur Cell: A Multifaceted Approach to Enhancing Cell Performance. Nano Lett. 13, 5891–5899 (2013).
Yu, Y. et al. Encapsulation of Sn@carbon Nanoparticles in Bamboo-like Hollow Carbon Nanofibers as an Anode Material in Lithium-Based Batteries. Angew. Chem. Int. Ed. 48, 6485–6489 (2009).
Luo, J. et al. Three-Dimensional Graphene Foam Supported Fe3O4 Lithium Battery Anodes with Long Cycle Life and High Rate Capability. Nano Lett. 13, 6136–6143 (2013).
Hu, L., Wu, F., Lin, C., Khlobystov, A. & Li, L. Graphene-modified LiFePO4 Cathode for Lithium Ion Battery beyond Theoretical Capacity. Nat. Commun. 4, 1687 (2013).
Pan, A., Wu, H., Yu, L. & Lou, X. Template-free Synthesis of VO2 Hollow Microspheres with Various Interiors and Their Conversion into V2O5 for Lithium-ion Batteries. Angew. Chem. Int. Ed. 52, 2226–2230 (2013).
Lou, X., Li, C. & Archer, L. Designed Synthesis of Coaxial SnO2@carbon Hollow Nanospheres for Highly Reversible Lithium Storage. Adv. Mater. 21, 2536–2539 (2009).
Lin, Y., Wei, T., Chien, H. & Lu, S. Manganese Oxide/Carbon Aerogel Composite: an Outstanding Supercapacitor Electrode Material. Adv. Energy Mater. 1, 901–907 (2011).
Viet, A., Reddy, M., Jose, R., Chowdari, B. & Ramakrishna, S. Nanostructured Nb2O5 Polymorphs by Electrospinning for Rechargeable Lithium Batteries. J. Phys. Chem. C 114, 664–671 (2010).
Jiang, Y., Wang, K., Zhang, H., Wang, J. & Chen, J. Hierarchical Li4Ti5O12/TiO2 Composite Tubes with Regular Structural Imperfection for Lithium Ion Storage. Scientific Reports 3, 3490 (2013).
Kim, J., Augustyn, V. & Dunn, B. The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5 . Adv. Eng. Mater. 2, 141–148 (2012).
Liu, C., Zhou, F. & Ozolins, V. First Principles Study for Lithium Intercalation and Diffusion Behavior in Orthorhombic Nb2O5 Electrochemical Supercapacitor. American Physical Society March Meeting, Abstr. B26.00003 (2012).
Augustyn, V. et al. High-Rate Electrochemical Energy Storage through Li+ Intercalation Pseudocapacitance. Nat. Mater. 12, 518–522 (2013).
Brezesinski, K. et al. Pseudocapacitive Contributions to Charge Storage in Highly Ordered Mesoporous Group V Transition Metal Oxides with Iso-Oriented Layered Nanocrystalline Domains. J. Am. Chem. Soc. 132, 6982–6990 (2010).
Wang, X. et al. High-Performance Supercapacitors Based on Nanocomposites of Nb2O5 Nanocrystals and Carbon Nanotubes. Adv. Eng. Mater. 1, 1089–1093 (2011).
Li, G., Wang, X. & Ma, X. Nb2O5-Carbon Core-Shell Nanocomposite as Anode Material for Lithium Ion Battery. J. Energy Chem. 22, 357–362 (2013).
Wei, M., Wei, K., Ichihara, M. & Zhou, H. Nb2O5 Nanobelts: A Lithium Intercalation Host with Large Capacity and High Rate Capability. Electrochem. Commun. 10, 980–983 (2008).
Sasidharan, M., Gunawardhana, N., Yoshio, M. & Nakashima, K. Nb2O5 Hollow Nanospheres as Anode Materials for Enhanced Performance in Lithium Ion Batteries. Mater. Res. Bull. 47, 2161–2164 (2012).
Kodama, R., Terada, Y., Nakai, I., Komaba, S. & Kumagai, N. Electrochemical and In situ XAFS-XRD Investigation of Nb2O5 for Rechargeable Lithium Batteries. J. Electrochem. Soc. 153, A583–588 (2006).
Zhang, G. & Lou, X. Controlled Growth of NiCo2O4 Nanorods and Ultrathin Nanosheets on Carbon Nanofibers for High-Performance Supercapacitors. Scientific Reports 3, 1470 (2013).
Yang, Q., Lu, Z., Sun, X. & Liu, J. Ultrathin Co3O4 Nanosheet Arrays with High Supercapacitive Performance. Scientific Reports 3, 3537 (2013).
Yuan, C. et al. Growth of Ultrathin Mesoporous Co3O4 Nanosheet Arrays on Ni Foam for High-Performance Electrochemical Capacitors. Energy Environ. Sci. 5, 7883–7887 (2012).
Hu, L. et al. CoMn2O4 Spinel Hierarchical Microspheres Assembled with Porous Nanosheets as Stable Anodes for Lithium-Ion Batteries. Scientific Reports 2, 986 (2012).
Li, Q. et al. Design and Synthesis of MnO2/Mn/MnO2 Sandwich-Structured Nanotube Arrays with High Supercapacitive Performance for Electrochemical Energy Storage. Nano Lett. 12, 3803–3807 (2012).
Li, H. et al. Amorphous Nickel Hydroxide Nanospheres with Ultrahigh Capacitance and Energy Density as Electrochemical Pseudocapacitor Materials. Nat. Commun. 4, 1894 (2012).
Lee, J., Hall, A., Kim, J. & Mallouk, T. A Facile and Template-free Hydrothermal Synthesis of Mn3O4 Nanorods on Graphene Sheets for Supercapacitor Electrodes with Long Cycle Stability. Chem. Mater. 24, 1158–1164 (2012).
Yang, S. et al. Bottom-up Approach toward Single-Crystalline VO2-Graphene Ribbons as Cathodes for Ultrafast Lithium Storage. Nano Lett. 13, 1596–1601 (2013).
Wang, Y., Zhong, Z., Chen, Y., Ng, C. T. & Lin, J. Controllable Synthesis of Co3O4 from Nanosize to Microsize with Large-Scale Exposure of Active Crystal Planes and Their Excellent Rate Capability in Supercapacitors Based on the Crystal Plane Effect. Nano Res. 4, 695–704 (2011).
Nan, C. et al. Size and Shape Control of LiFePO4 Nanocrystals for Better Lithium Ion Battery Cathode Materials. Nano Res. 6, 469–477 (2013).
Wang, Z. et al. Template-free Synthesis of 3D Nb3O7F Hierarchical Nanostructures and Enhanced Photocatalytic Activities. Phys. Chem. Chem. Phys. 15, 3249–3255 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51402345, 2014M551677, No. 21433013, No. 21403287) and Suzhou Science and Technology Development Program (No. ZXG2013002).
Author information
Authors and Affiliations
Contributions
M.N.L. designed and conducted the experiment. C.Y. and Y.G.Z. involved in the scientific discussions. M.N.L., C.Y. and Y.G.Z. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, M., Yan, C. & Zhang, Y. Fabrication of Nb2O5 Nanosheets for High-rate Lithium Ion Storage Applications. Sci Rep 5, 8326 (2015). https://doi.org/10.1038/srep08326
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08326
This article is cited by
-
Insights into the kinetics–morphology relationship of 1-, 2-, and 3D TiNb2O7 anodes for Li-ion storage
Nano Research (2023)
-
Nanostructuring versus microstructuring in battery electrodes
Nature Reviews Materials (2022)
-
Nanoscale niobium oxides anode for electrochemical lithium and sodium storage: a review of recent improvements
Journal of Nanostructure in Chemistry (2021)
-
Facile synthesis of flower-like T-Nb2O5 nanostructures as anode materials for lithium-ion battery
Journal of Materials Science: Materials in Electronics (2021)
-
Nanostructured T-Nb2O5-based composite with reduced graphene oxide for improved performance lithium-ion battery anode
Journal of Materials Science (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.