Abstract
IL-1 receptor accessory protein-like 1 (IL1RAPL1) is responsible for nonsyndromic intellectual disability and is associated with autism. IL1RAPL1 mediates excitatory synapse formation through trans-synaptic interaction with PTPδ. Here, we showed that the spine density of cortical neurons was significantly reduced in IL1RAPL1 knockout mice. The spatial reference and working memories and remote fear memory were mildly impaired in IL1RAPL1 knockout mice. Furthermore, the behavioural flexibility was slightly reduced in the T-maze test. Interestingly, the performance of IL1RAPL1 knockout mice in the rotarod test was significantly better than that of wild-type mice. Moreover, IL1RAPL1 knockout mice consistently exhibited high locomotor activity in all the tasks examined. In addition, open-space and height anxiety-like behaviours were decreased in IL1RAPL1 knockout mice. These results suggest that IL1RAPL1 ablation resulted in spine density decrease and affected not only learning but also behavioural flexibility, locomotor activity and anxiety.
Similar content being viewed by others
Introduction
Intellectual disability (ID), formerly referred as mental retardation, is a neurodevelopmental disorder affecting 1–3% of the population1. ID involves impairments of general mental abilities that impact adaptive functioning in conceptual, social and practical domains and is characterized by an intelligence quotient of 70 or below2. Genetic causes of ID are highly heterogeneous, but there may be some common pathways providing opportunities for the development of knowledge-based therapeutics1,3,4. Nonsyndromic ID is characterized by reduced cognitive function without any other clinical features, thus providing the most direct approach to specifically study the neurobiology of cognition and pathogenesis of ID.
IL-1 receptor accessory protein-like 1 (IL1RAPL1) is the product of an X-linked gene responsible for a nonsyndromic form of ID5,6,7,8,9,10,11,12,13. The IL1RAPL1 gene is also associated with autism spectrum disorders (ASDs)7,8,12. IL1RAPL1 contains three extracellular immunoglobulin (Ig)-like domains, a transmembrane domain and a Toll/IL-1 receptor (TIR) domain in the cytoplasmic portion and thus belongs to the IL-1 receptor family5,14,15. IL1RAPL1 is selectively expressed in the brain5,14. Previous studies showed that IL1RAPL1 regulates N-type voltage-gated calcium channel through the neuronal calcium sensor-116,17 and synaptic localization of PSD-95 by controlling c-Jun terminal kinase activity18. We found that IL1RAPL1 mediates synapse formation in zebrafish olfactory sensory neurons and mouse cortical neurons19,20. In mouse cortical neurons, postsynaptic IL1RAPL1 mediates excitatory synapse formation through trans-synaptic interaction with specific variants of presynaptic protein tyrosine phosphatase (PTP) δ20. Valnegri et al. reported that IL1RAPL1 regulates synapse formation by interacting with PTPδ and RhoGAP221. We thus proposed that the impairment of synapse formation underlies the pathogenesis of ID and ASDs in patients with IL1RAPL1 mutations20,22. IL1RAPL1 stimulates the increase of dendritic protrusions through the interaction of the TIR domain with Mcf2-like (Mcf2l)20,23. IL1RAPL1 also regulates the stabilization of glutamatergic synapses by controlling AMPA receptor trafficking through the Mcf2l-RhoA-ROCK signaling pathway23. Here, we generated IL1RAPL1 knockout mice on the pure C57BL/6 genetic background and examined the effects of IL1RAPL1 ablation on the cortical and hippocampal spine density and learning ability. Behavioural battery tests revealed several characteristic features of IL1RAPL1 knockout mice including impairments of learning and memory, reduced behavioural flexibility, enhanced locomotor activity and reduced anxiety-like behaviours.
Results
Spine density is decreased in IL1RAPL1 knockout mice
We generated IL1RAPL1 knockout mice using C57BL/6 embryonic stem cells24 (see Supplementary Fig. S1 online). The IL1RAPL1 knockout mice grew and mated normally. Western blot analysis confirmed the absence of IL1RAPL1 of ~85 kDa in the brain of the mutant mice (Fig. 1A). The size and proportion of the brain and the Nissl-staining patterns of the cerebral cortex and hippocampus of the mutant mice were comparable to those of the wild-type mice in agreement with a previous report25 (Fig. 1B and C).
Decreased spine density in IL1RAPL1 knockout mice.
(A) Western blot analysis of the immunoprecipitates of whole brain lysates from wild-type (Il1rapl1+/Y) and IL1RAPL1 knockout (Il1rapl1−/Y) mice with anti-IL1RAPL1 antiserum. (B) Nissl-stained parasagittal whole brain sections of Il1rapl1+/Y and Il1rapl1−/Y mice. (C) Nissl-stained sections of hippocampus and cerebral cortex of Il1rapl1+/Y and Il1rapl1−/Y mice. (D) Accumulation of Shank2 signals of cultured cortical neurons from Il1rapl1+/Y and Il1rapl1−/Y mice by beads conjugated with PTPδ-ECD-Fc or Fc protein. (E) Intensity of staining signals for Shank2 on the surface of beads conjugated with PTPδ-ECD-Fc or Fc protein (n = 11–12). (F) Decrease in spine density of basal dendrites of hippocampal CA1 and cortical layer 2/3 pyramidal neurons in IL1RAPL1 knockout mice. (G) Spine densities of basal dendrites of hippocampal CA1 and cortical layer 2/3 pyramidal neurons of wild-type (n = 47 and 43 neurons, respectively from 3 animals) and IL1RAPL1 knockout (n = 31 and 29 neurons, respectively from 2 animals) mice. All values represent mean ± SEM. * P < 0.05, ** P < 0.01 and *** P < 0.001, respectively; Tukey's test (E) or t-test (G). Scale bars represent 5 mm in (B), 1 mm in (C) and 5 μm in (D, F).
IL1RAPL1 mediates excitatory synapse formation through trans-synaptic interaction with PTPδ in cultured cortical neurons and the synaptogenic activity of IL1RAPL1 is abolished by the ablation of PTPδ20. Consistently, the extracellular domain of PTPδ coated on magnetic beads induced excitatory postsynaptic differentiation of cultured cortical neurons from wild-type mice as indicated by the accumulation of an excitatory postsynaptic scaffold protein Shank2 on the beads (Fig. 1D and E). When cultured cortical neurons were prepared from IL1RAPL1 knockout mice, the accumulation of Shank2 on the beads was decreased by ~50%. We then examined the spine density in IL1RAPL1 knockout mice at postnatal day (P) 20 by labelling cerebral cortical and hippocampal neurons with DiI, since the spine density of basal dendrites of cortical layer 2/3 pyramidal neurons reaches plateau at P20 (ref. 26) and both IL1RAPL1 and PTPδ are widely expressed in the mouse brain including the cerebral cortex and hippocampus5,27. The spine density of basal dendrites of cortical layer 2/3 pyramidal neurons was significantly reduced in IL1RAPL1 knockout mice (p = 0.0099, t-test) (Fig. 1F and G). Similarly, there was a significant difference in the spine density of hippocampal CA1 pyramidal neurons between wild-type and IL1RAPL1 knockout mice (p = 0.0008) as reported previously18. The extents of the decreases of dendritic spines in IL1RAPL1 knockout mice were comparable to those in PTPδ knockout mice20. These results suggest that the ablation of IL1RAPL1 resulted in the impairment of the excitatory synapse formation of cortical and hippocampal neurons in vivo.
Acquisition and retention of spatial reference memory are impaired in IL1RAPL1 knockout mice
As shown in Supplementary Table S1, physical characteristics of IL1RAPL1 knockout mice at the age of 11–14 weeks were comparable to those of wild-type mice except for a slight increase in body temperature. There were no significant differences in the hot plate, acoustic startle response and paired pulse inhibition tests between wild-type and IL1RAPL1 knockout mice.
We examined spatial reference memory by the Barnes maze test. Both wild-type and IL1RAPL1 knockout mice learned to locate the target hole during the course of the training period, as indicated by gradual reductions in the number of search errors and escape latency. However, there were significant differences in the number of errors and escape latency between wild-type and IL1RAPL1 knockout mice (number of errors; genotype effect, F1, 75 = 9.77, p = 0.003; genotype × block of trials effect, F4, 300 = 1.12, p = 0.3, repeated measures ANOVA: escape latency; genotype effect, F1, 75 = 7.55, p = 0.008; genotype × block of trials, F4, 300 = 0.36, p = 0.8) (Fig. 2A), suggesting an impairment in acquisition of spatial reference memory in IL1RAPL1 knockout mice. We conducted the first probe test 1 day after the last day of training and the second probe test 8 or 29 days after. In the probe test, both wild-type and IL1RAPL1 knockout mice located to the correct hole where the escape box had been and the time spent around the target hole was comparable between genotypes (the first probe test; F1, 75 = 0.093, p = 0.8: the second probe test 8 days after the last training; F1, .37 = 0.11, p = 0.7: the second probe test 29 days after; F1, .36 = 0.014, p = 0.9, one-way ANOVA) (Fig. 2B–D). In the first and second probe tests conducted 1 and 8 days after the last training, both wild-type and IL1RAPL1 knockout mice remembered the correct hole accurately (the first probe test; p = 0.1: the second probe test 8 days after the last training, p = 0.2, Mann-Whitney's u-test) (Fig. 2B and C). In contrast, in the second probe test conducted 29 days after the last training, the accuracy of spatial memory of IL1RAPL1 knockout mice was worse than that of wild-type mice (p = 0.02) (Fig. 2D). These results suggest that the retention of spatial reference memory was mildly impaired in IL1RAPL1 knockout mice.
Deficit of acquisition and retention of spatial reference memory in IL1RAPL1 knockout mice.
(A–D) Barnes maze test. (A) Number of errors to the first encounter of the escape hole (left panel) and escape latency (right panel) of wild-type (open circle, n = 39) and IL1RAPL1 knockout mice (closed circle, n = 38). Data are presented as average of 3 trials. (B) Time spent around each hole in the probe trial conducted 1 day after the last training (left panel) and accuracy of reference memory (right panel). White and black bars represent wild-type (n = 39) and IL1RAPL1 knockout mice (n = 38), respectively. (C, D) Time spent around each hole in the probe trial conducted 8 or 29 days after the last training (left panel) and accuracy of reference memory (right panel). White and black bars represent wild-type (n = 19 or 20) and IL1RAPL1 knockout mice (n = 19), respectively. The accuracy of reference memory was evaluated by the ratio of time spent around the target hole to that spent around holes at target and ±30 degree (B–D). (E, F) Reversal training in Barnes maze test. (E) Number of errors to the first encounter of the escape hole (left panel) and escape latency (right panel) of wild-type (open circle, n = 39) and IL1RAPL1 knockout mice (closed circle, n = 38). One day after the 12th training, the target hole was move to the opposite position. Data are presented as average of 3 trials. (F) Time spent around each hole in the probe trial conducted 1 day after the last training White and black bars represent wild-type (n = 39) and IL1RAPL1 knockout mice (n = 38), respectively. Time spent around the target hole and times spent around the hole at 180 degree were compared. All values represent as mean ± SEM. ** p < 0.01 and *** p < 0.001, respectively; Mann-Whitney's U–test (D), two-way repeated measures ANOVA followed by Fisher's LSD test (E) or paired t-test (F). The p values indicate genotype effect in two-way repeated measures ANOVA (A and E).
Spatial working memory is impaired in IL1RAPL1 knockout mice
We next examined the spatial working memory by the eight-arm radial maze and T-maze tests. Both tests were performed with food-restricted mice, using food pellets as a reward. In the eight-arm radial maze test, there were no significant differences between wild-type and IL1RAPL1 knockout mice in the number of different arm choices among the first 8 entries (genotype effect, F1, 37 = 1.87, p = 0.2; genotype × block of trials effect, F11, 407 = 0.83, p = 0.6, repeated measures ANOVA) (Fig. 3A) and the total number of revisiting errors in which the mice returned to the arms that had been visited previously to retrieve a food pellet (genotype effect, F1, 37 = 3.39, p = 0.07; genotype × block of trials effect, F11, 407 = 0.33, p = 0.98) (Fig. 3B). We then examined the eight-arm radial maze test with a delay time. The number of different arm choices was significantly smaller in IL1RAPL1 knockout mice than in wild-type mice (Fig. 3C: genotype effect, F1, 37 = 6.44, p = 0.02; genotype × block of trials effect, F2, 74 = 0.58, p = 0.6) and that of revisiting errors was significantly larger in knockout mice (Fig. 3D: genotype effect, F1, 37 = 4.94, p = 0.03; genotype × block of trials effect, F2, 74 = 0.003, p = 1.0). These results suggest that the spatial working memory was mildly impaired in IL1RAPL1 knockout mice.
Deficit of spatial working memory in IL1RAPL1 knockout mice.
(A–D) Eight-arm radial maze test of wild-type (open circles and white bars, n = 19) and IL1RAPL1 knockout mice (closed circles and black bars, n = 20). Different arm choices among the first 8 arms (A and C) and total number of arms revisited (B and D) were recorded. During 25–30th training, a delay was applied after the first 4 pellets were consumed (C and D). Data are presented as average of 2 trials. (E and F) The percentage of correct responses in T-maze forced alternation task of wild-type (open circles and white bars, n = 40) and IL1RAPL1 knockout mice (closed circles and black bars, n = 40). Delays were applied in order of 3, 3, 10, 30, 60, 3, 3, 10, 30 and 60 s per session (F). (G) The percentage of correct responses in T-maze left/right discrimination task of wild-type (open circles, n = 40) and IL1RAPL1 knockout mice (closed circles, n = 40). After session 10, the baited arm was changed to the other side. All values represent as mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, respectively; two-way repeated measures ANOVA followed by Fisher's LSD test (E and G). The p values indicate genotype effect in two-way repeated measures ANOVA (E and G).
In the T-maze forced alternation task, both wild-type and IL1RAPL1 knockout mice gradually improved their performance. However, IL1RAPL1 knockout mice showed considerably lower performance as indicated by the percentage of correct choices made throughout the training period than wild-type mice (genotype effect, F1, 78 = 17.6, p < 0.0001; genotype × session effect, F9, 702 = 2.02, p = 0.03) (Fig. 3E). Thus, the impairment of spatial working memory was observed also in the T-maze forced alternation task. We then applied a delay time as an intratrial interval. However, the percentage of correct choices was comparable between wild-type and IL1RAPL1 knockout mice (genotype effect, F1, 78 = 3.23, p = 0.08; genotype × delay time effect, F3, 234 = 0.64, p = 0.6) (Fig. 3F).
Long-term fear memories are impaired in IL1RAPL1 knockout mice
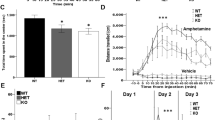
We further examined the cognitive functions of IL1RAPL1 knockout mice by the contextual and cued fear conditioning tests. During the conditioning period, freezing behaviour before the first presentation of cue–shock pairings was minimal and did not differ between wild-type and IL1RAPL1 knockout mice. After footshocks, freezing responses of both genotypes were increased, but the level of freezing of IL1RAPL1 knockout mice was significantly lower than that of wild-type mice (genotype effect, F1, 38 = 10.2, p = 0.003; genotype × time effect, F7, 266 = 7.97, p < 0.0001) (Fig. 4A). One day after conditioning, there was no significant difference in the level of freezing in the contextual test between wild-type and IL1RAPL1 knockout mice (F1, 38 = 3.08, p = 0.09) (Fig. 4B). However, the level of freezing of IL1RAPL1 knockout mice in the cued test was significantly lower than that of wild-type mice (genotype effect, F1, 38 = 19.9, p < 0.0001; genotype × time effect, F2, 76 = 6.90, p = 0.002) (Fig. 4C). Twenty-eight days after conditioning, the freezing levels of IL1RAPL1 knockout mice were significantly lower than those of wild-type mice in both the contextual and cued tests (contextual test; genotype effect, F1, 38 = 20.4, p < 0.0001; genotype × trial effect, F4, 152 = 0.49, p = 0.74: cued test; genotype effect, F1, 38 = 81.0, p < 0.0001; genotype × trial effect, F2, 76 = 14.6, p < 0.001) (Fig. 4D and E). Since the freezing levels on the conditioning day were different between two genotypes, we performed an analysis of covariance (ANCOVA) in which the average freezing level on the conditioning day (baseline freezing) was treated as a covariate. There were significant differences in cued tests conducted 1 day (pretone, F1,36 < 0.001, p = 0.99; after tone, F1,36 = 10.0, p = 0.003) and 28 days after conditioning (pretone, F1,36 = 0.81, p = 0.4; after tone, F1,36 = 13.9, p = 0.0006), while no significant differences were found in contextual tests conducted 1 day (F1,36 = 0.76, p = 0.4) and 28 days after conditioning (F1,36 = 3.37, p = 0.07). These results suggest that the remote cued fear memory was impaired in IL1RAPL1 knockout mice.
Impaired freezing responses of IL1RAPL1 knockout mice.
(A) Freezing responses on the conditioning day. (B) Freezing responses on the contextual test at 1 day after conditioning. (C) Freezing responses on the cue test at 1 day after conditioning. (D) Freezing responses on the contextual test at 28 days after conditioning. (E) Freezing responses on the cue test at 28 days after conditioning. Open and closed circles represent wild-type and IL1RAPL1 knockout mice (n = 20 each), respectively. Bold lines and arrows represent tone and footshock, respectively. All values represent as mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, respectively; two-way repeated measures ANOVA followed by Fisher's LSD test (A, C and E). The p values indicate genotype effect in two-way repeated measures ANOVA (A and C–E).
Motor learning
We also examined the motor function of IL1RAPL1 knockout mice. The grip strength was comparable between wild-type (1.3 ± 0.04 N) and IL1RAPL1 knockout mice (1.2 ± 0.03 N) (F1, 78 = 3.61, p = 0.06, one-way ANOVA). In the wire hang test, the time to fall of IL1RAPL1 knockout mice (26.8 ± 3.3 s) was significantly shorter than that of wild-type mice (39.6 ± 3.4 s) (F1, 78 = 7.42, p = 0.008), suggesting that the neuromuscular strength of the mutant mice was reduced. In the accelerated rotarod test, IL1RAPL1 knockout mice performed better than wild-type mice in the first trial (Fig. 5). In subsequent trials, both wild-type and IL1RAPL1 knockout mice showed gradual increases in retention time on the rotating rod. However, the latency to fall from the apparatus of IL1RAPL1 knockout mice was significantly longer than that of wild-type mice (genotype effect, F1, 38 = 46.7, p < 0.0001; genotype × trial effect, F5, 190 = 1.88, p = 0.1, repeated measures ANOVA). Thus, the motor coordination of IL1RAPL1 knockout mice was improved but the motor learning ability was comparable between two genotypes.
Rotarod performance of IL1RAPL1 knockout mice.
Time that mice remained on the rotating rod before falling was measured. Open and closed circles represent wild-type and IL1RAPL1 knockout mice (n = 20 each). All values represent as mean ± SEM. The p value indicates genotype effect in two-way repeated measures ANOVA.
Behavioural flexibility is reduced in IL1RAPL1 knockout mice
We examined behavioural flexibility using reversal tasks of the Barnes maze and T-maze left-right discrimination tests. In the Barnes maze test, mice were trained for additional 4 days after the second probe test. Then, the target was moved to the opposite site. During the reversal training, the number of errors and escape latency were significantly larger in IL1RAPL1 knockout mice than in wild-type mice (number of errors; genotype effect, F1, 75 = 14.3, p = 0.0003; genotype × block of trials effect, F2, 150 = 0.71, p = 0.5: escape latency; genotype effect, F1, 75 = 13.1, p = 0.0005; genotype × block of trials effect, F2, 150 = 1.11, p = 0.3) (Fig. 2E). In the probe test after the reversal training, the time spent around the target hole of IL1RAPL1 knockout mice was much shorter than that of wild-type mice (F1, 75 = 7.56, p = 0.007, one-way ANOVA). IL1RAPL1 knockout mice, however, stayed significantly longer in the new target position than in the original position, as wild-type mice did (wild-type, p < 0.0001; IL1RAPL1 KO, p < 0.0001, paired t-test) (Fig. 2F), Thus, IL1RAPL1 knockout mice exhibited normal behavioural flexibility in spite of the impaired acquisition of spatial reference memory.
In the T-maze left-right discrimination test, mice were trained to choose the baited arm that was fixed to one side. During the first 10 sessions, the percentage of correct responses of IL1RAPL1 knockout mice was slightly higher than that of wild-type mice (genotype effect, F1, 78 = 4.57, p = 0.04; genotype × session effect, F9, 702 = 0.61, p = 0.8, repeated measures ANOVA) (Fig. 3G). At the 11th session, the baited arm was changed to the other side and the mice were trained for 6 sessions. The percentage of correct choices during reversal sessions was significantly lower in IL1RAPL1 knockout mice than in wild-type mice (genotype effect, F1, 78 = 7.56, p = 0.007; genotype × session effect, F5, 390 = 4.03, p = 0.001) (Fig. 3G). Thus, the behavioural flexibility of IL1RAPL1 knockout mice was reduced in this test.
Social interaction is increased in IL1RAPL1 knockout mice
In the social interaction test in a novel environment, two mice from separate cages were placed together in a small chamber in which neither has established territory for 10 min. In this test, IL1RAPL1 knockout mice traveled significantly longer distances than wild-type mice (F1, 33 = 12.3, p = 0.001, one-way ANOVA) (Fig. 6A). Both the number of contacts and the total duration of active contacts of IL1RAPL1 knockout mice were also greater than those of wild-type mice (Fig. 6B; F1, 33 = 17.1, p = 0.0002: Fig. 6C; F1, 33 = 15.3, p = 0.0004). The total duration of contacts of IL1RAPL1 knockout mice was significantly longer than that of wild-type mice (F1, 33 = 16.8, p = 0.0003) (Fig. 6D) and there was no significant difference in the mean duration per contact between wild-type and IL1RAPL1 knockout mice (F1, 33 = 3.51, p = 0.07) (Fig. 6E). These results suggest that the social interaction of IL1RAPL1 knockout mice was increased.
Abnormal social interaction in IL1RAPL1 knockout mice.
(A–E) Social interaction test in a novel environment of wild-type mice (white bars, n = 17) and IL1RAPL1 knockout mice (black bars, n = 18). Distance traveled (A), number of contacts (B), total duration of active contacts (C), total duration of contacts (D) and mean duration per contacts (E) were recorded. (F and G) Three-chamber social interaction test of wild-type mice and IL1RAPL1 knockout mice (n = 40 each). A wire cage with a stranger mouse was placed in one side chamber and an empty wire cage was placed in opposite side chamber. Time spent around the wire cage (F) and time spent around the chamber (G) in were recorded. (H and I) Three-chamber social novelty preference test of wild-type mice and IL1RAPL1 knockout mice (n = 40 each). A novel stranger mouse was placed in a wire cage in one side chamber and a familiar mouse that was used in (F and G) was place in a wire cage in the opposite site. Time spent around the wire cage (H) and time spent around the chamber (I) were recorded. All values represent as mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001, respectively; One-way ANOVA (A–E) and paired t-test (F–I).
Next, we performed Crawley's three-chamber social interaction test, which consists of sociability test and preference for social novelty test. In the test of sociability, a wire cage with a stranger mouse was placed in one side chamber and an empty cage was placed in the other side chamber. While wild-type mice spent almost the same time around the wire cage of either side (p = 0.4, paired t-test), IL1RAPL1 knockout mice spent significantly longer time around the wire cage with the stranger mouse than around the empty wire cage (p = 0.0001) (Fig. 6F). Consistently, IL1RAPL1 knockout mice but not wild-type mice showed a preference for the chamber with the stranger mouse (wild-type, p = 0.9; IL1RAPL1 knockout, p < 0.0001) (Fig. 6G). These results suggest that social interactions were enhanced in IL1RAPL1 knockout mice. In the social novelty preference test, a second stranger mouse was introduced into the empty cage and the interactions of the mice with novel and familiar mice were compared. Both wild-type and IL1RAPL1 knockout mice spent longer time around the wire cage with the stranger mouse than around that with the familiar mouse (wild-type, p = 0.05; IL1RAPL1 knockout, p = 0.04) (Fig. 6H). There was no significant difference in the preference for the chamber with the stranger mouse between two genotypes (wild-type, p = 0.3; IL1RAPL1 knockout, p = 0.08) (Fig. 6I). In these sociability tests, wild-type mice exhibited no significant preference for stranger mouse. The time spent around the cage reflects the balance between tendencies to explore and avoid the unfamiliar, since the presentation of a novel object or novel environment normally elicits fear response or neophobia in animals. Thus, the tendency may shift toward avoidance behaviour in wild-type mice under the conditions employed28.
To examine the vocal behaviour of IL1RAPL1 knockout mice, we recorded ultrasonic vocalizations of individual male IL1RAPL1 knockout and wild-type mice upon contact with an ovariectomized female mouse. There were no significant differences in the latency to start calling and number of calls per session (see Supplementary Fig. S2A and B online). The duration of syllables and peak frequency of syllables were comparable between IL1RAPL1 knockout and wild-type mice while the average interval between syllables was slightly shorter in IL1RAPL1 knockout mice (p = 0.046, t-test) (see Supplementary Fig. S2C–E online). There were no significant differences in the probabilities of each categorized syllable occurrence (10 in total) between IL1RAPL1 knockout and wild-type mice (F9,14 = 0.54, p = 0.8, MANOVA) (see Supplementary Fig. S2F online). These results suggest no obvious deficit in vocal communications in IL1RAPL1 knockout mice.
Enhanced locomotor activity and reduced anxiety-like behaviours of IL1RAPL1 knockout mice
We examined the locomotor activity and anxiety-like behaviour in the open field, the elevated plus maze and the light/dark transition tests. IL1RAPL1 knockout mice consistently exhibited greater locomotor activity in all of the tasks we examined. Total distances traveled by IL1RAPL1 knockout mice were significantly longer than those of wild-type mice in the open field test (F1, 78 = 8.04, p = 0.006, repeated measures ANOVA) (Fig. 7A), the elevated plus maze test (F1, 78 = 23.5, p < 0.0001, one-way ANOVA) (Fig. 7E) and the light/dark transition test (light, F1, 78 = 4.75, p = 0.03; dark, F1, 78 = 7.74, p = 0.007) (Fig. 7I). In addition, there were significant differences between wild-type and IL1RAPL1 knockout mice in the stereotypic counts in the open field test (F1, 78 = 5.67, p = 0.02, repeated measures ANOVA) (Fig. 7C) and in the number of total entries in the elevated plus maze test (F1, 78 = 18.1, p < 0.0001, one-way ANOVA) (Fig. 7F). Vertical activity in the open field (F1, 78 = 0.16, p = 0.7, repeated measures ANOVA) (Fig. 7B) and the number of transitions in the light–dark transition test (F1, 78 = 0.89, p = 0.3, one-way ANOVA) (Fig. 7K) did not differ between wild-type and IL1RAPL1 knockout mice.
Increased locomotor activity and decreased anxiety-like behaviour of IL1RAPL1 knockout mice.
(A–D) Open field test of wild-type mice (open circles, n = 40) and IL1RAPL1 knockout mice (closed circles, n = 40). Total distance (A), vertical activity (B), stereotypic counts (C) and time spent in center arena (D) were scored in each 5 min period. (E–H) Elevated plus maze test of wild-type mice (white bars, n = 40) and IL1RAPL1 knockout mice (black bars, n = 40). Total distance traveled (E), number of entries into arms (F), percentage of time spent on the open arms (G) and percentage of entries into the open arms (H) were recorded. (I–L) Light/dark transition test of wild-type mice (white bars, n = 40) and IL1RAPL1 knockout mice (black bars, n = 40). Total distance traveled in the light and dark chambers (I), time spent in the light chamber (J), number of light/dark transition (K) and first latency to enter the light chamber (L) were recorded. All values represent as mean ± SEM.* p < 0.05, ** p < 0.01 and *** p < 0.001, respectively; Two-way repeated measures ANOVA followed by Fisher's LSD test (A, C, D) and one-way ANOVA (E–L). The p values indicate genotype effect in two-way repeated measures ANOVA (A, C, D).
In the open field test, mice tend to walk in the periphery of the open field arena, a behaviour called thigmotaxis. Increase of time spent in the central area is usually considered to reflect anxiolysis. IL1RAPL1 knockout mice spent significantly longer time in the central field of the open field arena than wild-type mice (F1, 78 = 11.2, p = 0.001, repeated measures ANOVA) (Fig. 7D). The elevated plus maze test is based on the natural aversion of mice to open and elevated spaces. IL1RAPL1 knockout mice spent significantly longer time in the open arms than wild-type mice (F1, 78 = 19.1, p < 0.0001, one-way ANOVA) (Fig. 7G). The percentage of entries into open arms was significantly higher in IL1RAPL1 knockout mice than in wild-type mice (F1, 78 = 16.6, p = 0.0001) (Fig. 7H). In addition to an aversion to elevated open spaces, mice also have a natural aversion to brightly illuminated spaces, which is reflected in their behaviour when subjected to the light/dark transition test. In this test, there were no significant differences between wild-type and IL1RAPL1 knockout mice in the time spent in the light chamber (F1, 78 = 2.68, p = 0.1) (Fig. 7J) and the first latency to enter the light chamber (F1, 78 = 0.12, p = 0.7) (Fig. 7L). Thus, anxiety-like behaviours of IL1RAPL1 knockout mice were reduced in the open field test and the elevated plus maze test, but not in the light/dark transition test.
Discussion
IL1RAPL1 mediates excitatory but not inhibitory synapse formation of cultured cortical neurons by trans-synaptic interaction with PTPδ20,21. Although the synaptogenic activity of IL1RAPL1 was completely abolished in PTPδ knockout mice20, the activity of PTPδ to induce excitatory postsynaptic differentiation was reduced to ~50% in cultured cortical neurons from IL1RAPL1 knockout mice. Thus, IL1RAPL1 mediates synapse formation solely through presynaptic PTPδ, while PTPδ organizes postsynaptic differentiation by the interaction with IL1RAPL1 and other proteins. The residual postsynapse-inducing activity of PTPδ in IL1RAPL1 knockout neurons may be ascribed to mutiple postsynaptic adhesion molcules including IL-1RAcP, Slitrks and netrin-G ligand-3, although the quantitative contributions of these molecules to PTPδ-mediated postsynaptic differentiation remain to be examined27,29,30,31,32. Corresponding to the loss of IL1RAPL1-mediated excitatory synapse formation, we showed that the density of dendritic spines was significantly reduced in the cerebral cortex and hippocampus of IL1RAPL1 knockout mice. Similarly, the decrease of dendritic spines but not inhibitory synapses was observed in the CA1 region of the hippocampus of IL1RAPL1 knockout mice18. High frequency stimulation-induced LTP at Schaffer collateral-CA1 pathway was unchanged in hippocampal slices of IL1RAPL1 knockout mice, while theta-burst-induced LTP was slightly reduced18. Wild-type and IL1RAPL1 knockout mice exhibited similar levels of LTP at thalamo-lateral amygdala synapses in acute slices, but fear-induced LTP occlusion was only partial in IL1RAPL1 knockout mice, probably because of a lower LTP induction in vivo during fear acquisition33. Selective impairment of excitatory synapse formation may lead to an excitation and inhibition imbalance and impairment of LTP induction in vivo.
We found that IL1RAPL1 knockout mice consistently showed impairments of cognitive functions in several learning tests. In the Barnes maze test, the number of errors of the mutant mice during the training period was significantly larger than that of wild-type mice. On the probe test conducted 29 days after the last training, IL1RAPL1 knockout mice spent comparable time around the correct and adjacent holes, while on the probe test conducted 1 day or 8 days after the last training, IL1RAPL1 knockout mice located to the correct hole as wild-type mice did. These results suggest that the acquisition of spatial reference memory is slower in IL1RAPL1 knockout mice than in wild-type mice, but the mutant mice can perform the task if they are trained for long enough. Furthermore, IL1RAPL1 knockout mice have a difficulty to retain the remote memory. The impairment of spatial working memory in IL1RAPL1 knockout mice shows similar tendency. In the eight-arm maze and T-maze forced alternation tests, the scores of IL1RAPL1 knockout mice were significantly lower than those of wild-type mice in the first few days but became comparable with those of wild-type mice at the end of the training period. When applied a delay, the scores of the mutant mice became worse than those of wild-type mice. Thus, IL1RAPL1 knockout mice have a difficulty to retain the spatial working memory. The learning deficiencies and memory declines observed in IL1RAPL1 knockout mice mimic the symptoms of ID children with IL1RAPL1 mutations. ID children with deletions in the IL1RAPL1 gene have slow developmental milestones such as the onsets of walking and speech and require special education9,10,11,13.
The IL1RAPL1 gene is also associated with ASDs7,8,12. ASDs are characterized by impairments in appropriate reciprocal social interactions, impairments in verbal social communication and high levels of ritualistic repetitive behaviours34. We found that the stereotypic counts of IL1RAPL1 knockout mice were increased in the open field test. The behavioural flexibility of IL1RAPL1 knockout mice was slightly reduced since the reduction was detected only in early sessions in the reversal task of the T-maze left-right discrimination test but not in that of the Barnes maze test. Interestingly, the performance of IL1RAPL1 knockout mice in the rotarod test was significantly better than that of wild-type mice, which may be interpreted that IL1RAPL1 knockout mice prone to be stereotypic in behaviour since better performance in repetitive test of motor coordination was also reported for mutant mice exhibiting autistic behaviour35,36. However, the social interaction of IL1RAPL1 knockout mice was increased in one-chamber and Crawley's three-chamber social interaction tests under the conditions employed. In addition, vocal communications of IL1RAPL1 knockout mice were comparable to those of wild-type mice.
Another characteristic feature of IL1RAPL1 knockout mice is an enhanced locomotor activity. IL1RAPL1 knockout mice consistently exhibited increases in locomotor activity in the open field, elevated plus maze, social interaction and light/dark transition tests. Thus, hyperactive behaviour reported for ID patients with mutations in the IL1RAPL1 gene9,10,11,13 was reproduced in IL1RAPL1 knockout mice. It is possible that locomotor activity may affect freezing levels of mice in fear conditioning tests although the distance traveled was comparable between IL1RAPL1 knockout mice and wild-type mice on the conditioning before tone-shock presentation (wild-type, 403.6 ± 19.8 cm, n = 20; IL1RAPL1 knockout, 425.2 ± 36.4 cm, n = 20; p = 0.6) in agreement with a previous report33. Further complication in fear conditioning tests was a difference in the freezing level during conditioning between two genotypes. Decreases of freezing levels in cued and contextual recall tests suggested the deficits in fear memories of IL1RAPL1 knockout mice in agreement with other reports33,37, but a significant difference was found only in cued tests by ANCOVA in our study. In the social interaction test, hyperactivity may increase the number of contacts in IL1RAPL1 knockout mice, while the mean duration per contact was comparable between genotypes. It is unlikely that locomotor activity would strongly affect the indices employed in spatial and working memory tests38,39.
We also noted that anxiety was reduced in IL1RAPL1 knockout mice. To measure anxiety-like behaviour in mice, open field, elevated plus maze and light/dark transition tests are widely used40. In the open field test, IL1RAPL1 knockout mice spent much longer time in the center of open field arena than wild-type mice. In the elevated plus maze test, the percentages of time on open arms and entry into open arms in IL1RAPL1 knockout mice were significantly higher than those of wild-type mice. Thus, anxiety-like behaviours of IL1RAPL1 knockout mice are consistently reduced in these two tests. On the other hands, time spent in light chamber and latency to enter the light chamber were comparable between two genotypes in the light/dark transition test. These results suggest that open-space and height anxieties were decreased in IL1RAPL1 knockout mice but bright-space anxiety was unaltered.
Present study with mutant mice on the pure C57BL/6 genetic background revealed that the ablation of IL1RAPL1 affects diverse brain functions including learning, memory, behavioural flexibility, locomotor activity and anxiety. Decrease of spine density in IL1RAPL1 mutant mice will cause excitation and inhibition imbalances in many brain circuits, since IL1RAPL1 is widely expressed in the brain5,33. Thus, it is reasonable that multiple brain functions are affected by the mutation. Human patients with IL1RAPL1 mutations are classified as ID and/or ASDs5,6,7,8,9,10,11,12,13. It remains to be examined how neural circuits responsible for these mental disorders are mainly affected by IL1RAPL1 mutations. Interestingly, model mice of Fragile X syndrome, the most common ID, show decreased anxiety, increased locomotor activity and mild or no deficits in spatial learning41,42,43. Based on the fact that FMRP regulates activity-dependent local mRNA translation upon metabotropic glutamate receptor 5 (mGluR5) stimulation, trials for the treatment of Fragile X syndrome using mGluR5 antagonists have begun (http://www.clinicaltrials.gov/). An increasing number of trials focus on treatment of the underlying defect, via re-equilibration of the biochemical imbalance that results from genetic mutations44. IL1RAPL1 induces excitatory synapse formation by trans-synaptic interaction with PTPδ20,21 and controls AMPA receptor trafficking by interacting with Mcf2l23. The decrease of spine density will cause the imbalance of excitation and inhibition in multiple brain circuits. Further investigation of the molecular mechanism of IL1RAPL1-mediated excitatory synapse formation would identify potential drug targets and IL1RAPL1 knockout mice will be useful to assess the new possible treatments.
Methods
Western blotting
Brain extracts from wild-type and IL1RAPL1 knockout mice were incubated with rabbit anti-IL1RAPL1 antiserum (custom-made antiserum, Sigma-Aldrich, St. Louis, MO, USA) overnight followed with Protein G-Sepharose (GE Healthcare, Little Chalfont, Buckinghamshire, UK) for 2 h. Immunoprecipitates were extensively washed, separated by SDS-PAGE and blotted with goat anti-IL1RAPL1 (R&D Systems, Minneapolis, MN, USA).
Nissl staining
Under deep pentobarbital anesthesia (100 μg/g of body weight, i.p.), mice were perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline. The sagittal sections (50 μm in thickness) were stained with 0.1% cresyl violet.
Synaptogenic assay
Fc and the extracellular domain of PTPδ fused to Fc (PTPδ-ECD-Fc) were expressed, purified and bound to Protein A-conjugated magnetic particles (smooth surface, 4.0–4.5 μm diameter; Spherotech, Libertyville, IL, USA) as previously described20. Beads coupled with Fc or PTPδ-ECD-Fc were added to cultured cortical neurons at days in vitro 14. After 24 h, cultures were fixed for immunostaining with rabbit anti-Shank2 antibody (1:200; Frontier Institute, Ishikari, Hokkaido, Japan). Image acquisition and quantification of Shank2 immunostaining signals were performed as described30.
DiI labeling
DiI labeling was performed essentially as described20,45. Coronal brain slices of 0.2-mm thickness containing the somatosensory cortex and hippocampus (typically 0.6 mm to 1.0 mm from the anterior edge of the hippocampus) were used for DiI labeling. Solid DiI crystals were applied onto the layer 2/3 of the somatosensory cortex (mediolaterally 2.0 to 3.5 mm from midline) and the pyramidal cell layer of the hippocampal CA1 region of the same coronal brain slices. The slices were incubated for 12 h and fixed for confocal microscopy. Basal dendrites of the pyramidal neurons in the cortical layer 2/3 and the hippocampal CA1 region were randomly sampled and imaged. Spines on dendrites 33–79 μm from their tips were identified and counted in the 3D projection images in a blind manner with respect to the genotype. When dendritic spines were too crowded to separate them from each other, we turned to serial stack images to delineate individual spines. By scrolling through the stack of different optical sections, individual spine heads could be identified. All dendritic protrusions with a clearly recognizable neck were counted as spines.
Animals and design of behavioural experiments
All experiments were performed in accordance with relevant guidelines and regulations. Wild-type and IL1RAPL1 knockout mice were generated by crossing of wild-type male mice and heterozygous female mice. All behavioural tests were carried out with male mice that were 11–14 weeks old at the start of testing. Mice were housed 4 (two pairs of wild-type and IL1RAPL1 knockout mice) per cage in a room with a 12 h light/dark cycle (lights on at 7:00 a.m.) with access to food and water ad libitum except for the period during which the T-maze and eight-arm radial maze tests were conducted. Behavioural testing was performed between 9:00 a.m. and 6:00 p.m. expect for the Barnes maze test which was performed between 8:00 p.m. and 4:00 a.m. Prior to all experiments, mice were left undisturbed in the testing room for at least 30 min to allow acclimation. After each test, the apparatus were cleaned with hypochlorous water to prevent a bias due to olfactory cues.
We prepared two independent groups of mice for behavioural testing except for ultrasonic vocalization test. The order of tests was as follows; the first group (wild-type and IL1RAPL1 knockout mice; n = 20 each): the general health and neurological screen, neuromuscular examination, light/dark transition test, open field test, elevated plus maze test, hot plate test, social interaction test in a novel environment, rotarod test, Crawley's sociability and preference for social novelty test, startle response/prepulse inhibition test, Barnes maze test, T-maze test, eight-arm radial maze test and contextual and cued fear conditioning test; the second group (wild-type and IL1RAPL1 knockout mice; n = 20 each): the general health and neurological screen, neuromuscular examination, light/dark transition test, open field test, elevated plus maze test, social interaction test in a novel environment, Crawley's sociability and preference for social novelty test, Barnes maze test, T-maze test. Each behavioural test was separated from each other at least by 1 day. All behavioural tests were conducted essentially as previously described39,46,47,48. Results of hot plate and startle response/prepulse inhibition tests are described in supplementary information. Ultrasonic vocalization test was conducted using a separate group of mice (see supplementary methods). All experiments were approved by the Animal Care and the Use Committees of Graduate School of Medicine, the University of Tokyo (Approval #1721T062), the National Institute for Physiological Sciences (Approval #13D056) and Azabu University (Approval #130226-4). Raw data from the behavioural tests, the date on which each experiment was performed and the age of the mice at the time of the experiment are available in the Mouse Phenotype Database (http://www.mouse-phenotype.org/).
Neuromuscular examination
Neuromuscular strength was examined by the grip strength test and wire hang test as described46. A grip strength meter (O'Hara & Co., Tokyo, Japan) was used to assess forelimb grip strength. Each mouse was tested three times and the greatest value measured was used for statistical analysis. In the wire hang test, a box (21.5 × 22 × 23 cm) with a wire mesh grid (10 × 10 cm) on its top (O'Hara & Co.) was used. Latency to fall was recorded, with a 60 s cutoff time.
Rotarod test
Rotarod test was conducted using an accelerating rotarod apparatus (UGO Basile, Comerio-Varese, Italy) as described39. Mice were placed on rotating drum and the latency to fall was recorded with 300 s cutoff. The speed of the drum accelerated from 4 to 40 rpm over a 5 min period. The mice were given 3 trials per day for 2 consecutive days.
Barnes maze test
The Barnes maze test was conducted on a white circular platform, 1.0 m in diameter, with 12 holes equally spaced around the perimeter (O' Hara & Co.), essentially as previously described47. Prior to beginning test, the mice completed one habituation trial to become familiar with the maze and the existence of the escape box. In the test, the trial ended when the mouse entered the escape box or after 5 min elapsed. Three trials per day were conducted for 5 consecutive days. On day 6, the mice received a probe trial conducted without the escape box for 3 min to confirm that this spatial task was acquired based on navigation by distal environment room cues. Mice were left undisturbed until receiving next probe trials. On day 13 or 34, mice once again received a probe trial to check remote memory. A single training trial was conducted immediately after each probe trial. As for a reversal task, the target was moved to a new position opposite to the original after the training trials for another 4 consecutive days. The mice were trained for 3 consecutive days and received a probe trial.
Eight-arm radial maze test
Eight-arm radial maze test was conducted using an automated eight-arm radial maze apparatus (O'Hara & Co.), essentially as previously described46. One week before the pre-training, mice were deprived of food until their body weight was reduced to 80–85% of the initial level. Mice were kept on a maintenance diet throughout the test. Before starting trials, all mice underwent the habituation to the apparatus and pre-training sessions to consume the pellet from the food well. One day after the pre-training sessions were complete, actual maze acquisition trials were performed. The mice went through one or two trials per day (18 trials total). During the 13–14th trial, a 30 s delay was initiated after four pellets had been taken by confining the mice in the center of platform. During the 15–16th and 17–18th trials, the delay period was extended to 2 min and 5 min, respectively.
T-maze test
Forced alternation task and left-right discrimination task were conducted using an automated T-maze apparatus (O' Hara & Co.), essentially as previously described49. After dieting, all mice underwent the habituation to the apparatus and pre-training sessions to consume the pellet from the food dispenser. One day after the pre-training sessions were complete, mice were subjected to a forced alternation task for 13 days (one session consisting of 10 trials per day; cutoff time, 50 min). The mice were confined to the start box for 3 s between trials and intratrial intervals between sample and choice runs were set at 3 s. On day 11, intratrial intervals between sample and choice runs were set at 10, 30 and 60 s to set higher demands on working memory. Next, the mice were subjected to a left-right discrimination task for 8 days (one session consisting of 10 trials, 2 sessions per day; cutoff time, 50 min). The mice were able to freely choose either the right or left arm of the T-maze. The baited (correct) arm was assigned to each mouse randomly. On day 6, the correct arm was changed to the opposite for reversal learning.
Contextual and cued fear conditioning
Contextual and cued fear conditioning tests were conducted as described48. A 55 dB white noise, which served as the conditioned stimulus (CS), was played for 30 s. During the last 2 s of the tone, a footshock (0.3 mA) was delivered as the unconditioned stimulus (US). Each mouse received three CS–US pairings with 2 min interstimulus interval. Contextual testing was conducted 24 h after conditioning. The mice were monitored for freezing for 5 min in the same chamber and then returned to their home cages. Cued testing with altered context was conducted 3 h after context test using a triangular box (35 × 35 × 40 cm) made of white opaque Plexiglas, which was located in a different room. Freezing behaviour was assessed during a 3 min free exploration, followed by a 3 min presentation of the tone.
Social interaction test in a novel environment (one-chamber)
Social interaction test was conducted as described48. Two mice of identical genotypes that were previously housed in different cages were placed in a box together (40 × 40 × 30 cm) and allowed to explore freely for 10 min. Images were captured at 1 frame per second and distance traveled between two successive frames was calculated for each mouse. The distance traveled was an average of those of two mice. If the two mice contacted each other and the distance traveled by either mouse was longer than 10 cm, the behavior was considered as ‘active contact’.
Crawley's sociability and preference for social novelty test
The test for sociability and preference for social novelty was conducted as described50,51. One day before testing, the subject mice were individually placed in the middle chamber and allowed to freely explore the entire apparatus for 10 min. In sociability test, an unfamiliar C57BL/6J male (stranger 1) that had no prior contact with the subject mouse was placed in one of the side chambers. The subject mouse was first placed in the middle chamber and allowed to explore the three chambers for 10 min. At the end of the first 10 min, each mouse was tested in a second 10-min session to quantitate social preference for a new stranger. A second, unfamiliar mouse was placed in the chamber that had been empty during the first 10-min session. This second stranger was enclosed in an identical small wire cage. The test mouse had a choice between the first, already-investigated unfamiliar mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). The stranger mice used in this experiment were 8 to 12-week-old C57BL/6J male mice, not littermates that had previously been habituated to placement in the small circular wire cage.
Open field test
Locomotor activity was measured in an open field apparatus (40 × 40 × 30 cm; Accuscan Instruments, Columbus, OH, USA) as described48. Total distance traveled, vertical activity (rearing measured by counting the number of photobeam interruptions), time spent in the center (20 × 20 cm) of the open field area and the stereotypic counts were recorded using VersaMax system (Accuscan Instruments). Data were collected for 120 min.
Elevated plus maze test
The elevated plus-maze test was conducted as described52. Mouse behaviour was recorded during a 10 min test period. The number of entries into and the time spent on open and enclosed arms were recorded.
Light/dark transition test
Light/dark transition test was conducted as reported53. Mice were placed into the dark side and allowed to move freely between the two chambers with door open for 10 min. The total number of transitions between chambers, time spent in each side, first latency to enter the light side and distance traveled in each chamber were recorded.
Image analysis
All applications used for the behavioural studies were based on NIH Image or Image J program and were modified as required39,49,52,53,54. ImageLD, ImageEP, ImageTM, ImageFZ are freely available following URL: http://www.mouse-phenotype.org/software.html.
Statistical analysis
Analysis was conducted using StatView (SAS Institute, Cary, NC, USA) or SPSS (IBM, Chicago, IL, USA). Data were analyzed by one-way ANOVA followed by Tukey's test, two-way repeated measures ANOVA followed by Fisher's LSD test, ANCOVA, MANOVA, Student's t test, paired t-test or Mann-Whitney's u-test. Statistical significance was assumed when p < 0.05.
References
Kaufman, L., Ayub, M. & Vincent, J. B. The genetic basis of non-syndromic intellectual disability: a review. J. Neurodevelop. Disord. 2, 182–209 (2010).
American Psychiatric Association. [Neurodevelopmental Disorders]. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. [33–41] (American Psychiatric Association, Arlington, VA. 2013).
van Bokhoven, H. Genetic and epigenetic networks in intellectual disability. Annu. Rev. Genet. 45, 81–104 (2011).
Ellison, J. W., Rosenfeld, J. A. & Shaffer, L. G. Genetic basis of intellectual disability. Annu. Rev. Med. 64, 441–450 (2013).
Carrié, A. et al. A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat. Genet. 23, 25–31 (1999).
Tabolacci, E. et al. A truncating mutation in the IL1RAPL1 gene is responsible for X-linked mental retardation in the MRX21 family. Am. J. Med. Genet. A 140, 482–487 (2006).
Bhat, S. S. et al. Disruption of the IL1RAPL1 gene associated with a pericentromeric inversion of the X chromosome in a patient with mental retardation and autism. Clin. Genet. 73, 94–96 (2008).
Piton, A. et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Hum. Mol. Genet. 17, 3965–3974 (2008).
Nawara, M. et al. Novel mutation of IL1RAPL1 gene in a nonspecific X-linked mental retardation (MRX) family. Am. J. Med. Genet. Part A 146A, 3167–3172 (2008).
Behnecke, A. et al. Intragenic deletion of IL1RAPL1: report of two cases and review of the literature. Am. J. Med. Genet. Part A 155, 372–379 (2011).
Franek, K. J. et al. Deletion of the immunoglobulin domain of IL1RAPL1 results in nonsyndromic X-linked intellectual disability associated with behavioral problems and mild dysmorphism. Am. J. Med. Genet. Part A 155, 1109–1114 (2011).
Youngs, E. L., Henkhaus, R., Hellings, J. A. & Butler, M. G. IL1RAPL1 gene deletion as a cause of X-linked intellectual disability and dysmorphic features. Eur. J. Med. Genet. 55, 32–36 (2012).
Barone, C. et al. Intragenic ILRAPL1 deletion in a male patient with intellectual disability, mild dysmorphic signs, deafness and behavioral problems. Am. J. Med. Genet. Part A 161A, 1381–1385 (2013).
Born, T. L. et al. Identification and characterization of two members of a novel class of the interleukin-1 receptor (IL-1R) family: delineation of a new class of IL-1R-related proteins based on signaling. J. Biol. Chem. 275, 29946–29945 (2000).
Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Bahi, N. et al. IL1 receptor accessory protein like, a protein involved in X-linked mental retardation, interacts with neuronal calcium sensor-1 and regulates exocytosis. Hum. Mol. Genet. 12, 1415–1425 (2003).
Gambino, F. et al. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. Proc. Natl. Acad. Sci. USA 104, 9063–9068 (2007).
Pavlowsky, A. et al. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr. Biol. 20, 103–115 (2010).
Yoshida, T. & Mishina, M. Zebrafish orthologue of mental retardation protein IL1RAPL1 regulates presynaptic differentiation. Mol. Cell Neurosci. 39, 218–228 (2008).
Yoshida, T. et al. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase δ. J. Neurosci. 31, 13485–13499 (2011).
Valnegri, P. et al. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPδ and RhoGAP2. Hum. Mol. Genet. 20, 4797–4809 (2011).
Mishina, M., Yoshida, T., Yasumura, M. & Uemura, T. Cortical Development: Neural Diversity and Neocortical Organization (eds Kageyama R., & Yamamori T.) 229–247 (Springer Japan, 2013).
Hayashi, T., Yoshida, T., Ra, M., Taguchi, R. & Mishina, M. IL1RAPL1 associated with mental retardation and autism regulates the formation and stabilization of glutamatergic synapses of cortical neurons through RhoA signaling pathway. PLos One 8, e56254. 10.1371/journal.pone.0066254 (2013).
Mishina, M. & Sakimura, K. Conditional gene targeting on the pure C57BL/6 genetic background. Neurosci. Res. 58, 105–112 (2007).
Gambino, F. et al. IL1RAPL1 controls inhibitory networks during cerebellar development in mice. Eur. J. Neurosci. 30, 1476–1486 (2009).
Ultanir, S. K. et al. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc. Natl. Acad. Sci. USA 104, 19553–19558 (2007).
Kwon, S. K., Woo, J., Kim, S. Y., Kim, H. & Kim, E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatase LAR, protein-tyrosine phosphatase δ (PTPδ) and PTPσ via specific domains regulate exciatory synapse formation. J. Biol. Chem. 285, 13966–13978 (2010).
Hattori, S. et al. Comprehensive behavioral analysis of pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice. Front. Behav. Neurosci. 6, 58. 10.3389/fnbeh.2012.00058 (2012).
Woo, J. et al. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat. Neurosci. 12, 428–437 (2009).
Yoshida, T. et al. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J. Neurosci. 32, 2588–2600 (2012).
Takahashi, H. et al. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat. Neurosci. 15, 389–398 (2012).
Yim, Y. S. et al. Slitrks control excitatory and inhibitory synapse formation with LAR receptor tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 110, 4057–4062 (2013).
Houbaert, X. et al. Target-specific vulnerability of excitatory synapses leads to deficits in associative memory in a model of intellectual disorder. J. Neurosci. 33, 13805–13819 (2013).
Crawley, J. N. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 10, 248–258 (2004).
Kwon, C. H. et al. Pten regulate neuronal arborization and social interaction in mice. Neuron 50, 377–388 (2006).
Nakatani, J. et al. Abnormal behavior in a chromosome- engineered mouse model for human 15q11-13 duplicaion seen in autism. Cell 137, 1235–1246 (2009).
Zhang, C. L. et al. The hippocampo-amygdala control of contextual fear expression is affected in a model of intellectual disability. Brain Struct. Funct. 10.1007/s00429-014-0882-x (2014).
Barnes, C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74–104 (1979).
Miyakawa, T., Yamada, M., Duttaroy, A. & Wess, J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 21, 5239–5250 (2001).
Crawley, J. N. [Emotional Behaviors: Animal Models of Psychiatric Diseases]. What's Wrong with My Mouse? [238–245] (Willy-Liss, New York 2007).
Peier, A. M. et al. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Human Mol. Genet. 9, 1145–1159 (2000).
Mineur, Y. S., Sluyter, F., de Wit, S., Oostra, B. A. & Crusio, W. E. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus 12, 39–46 (2002).
Eadie, B. D. et al. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol. Dis. 36, 361–373 (2009).
Picker, J. D. & Walsh, C. A. New innovations: therapeutic opportunities for intellectual disabilities. Ann. Neurol. 74, 382–390 (2013).
Kim, B. G., Dai, H. N., McAtee, M., Vicini, S. & Bregman, B. S. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp. Neurol. 198, 401–415 (2006).
Arron, J. R. et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 441, 595–600 (2006).
Miyakawa, T. et al. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus 11, 763–775 (2001).
Ihara, M. et al. Sept4, a component of presynaptic scaffold and lewy bodies is required for the suppression of α-synuclein neurotoxicity. Neuron 53, 519–533 (2007).
Shoji, H., Hagihara, H., Takao, K., Hattori, S. & Miyakawa, T. T-maze forced alternation and left-right discrimination tasks for assessing working and reference memory in mice. J. Vis. Exp. 60, e3300, 10.3791/3300 (2012).
Moy, S. S. et al. Sociability and preference for social novelt in five inbred strains: an approach to assess autistic-like behavior in mice. Genes. Brain Behav. 3, 287–302 (2004).
Tanda, K. et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory and increased D1-mediated dopaminergic signaling in neuronal nitric ixide synthase knockout mice. Mol. Brain 2, 19 (2009).
Komada, M., Takao, K. & Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 22, e1088, 10.3791/1088 (2008).
Takao, K. & Miyakawa, T. Light/dark transition test for mice. J. Vis. Exp. 13, e104, 10.3791/104 (2006).
Shoji, H., Takao, K., Hattori, S. & Miyakawa, T. Contextual and cued fear conditioning test using a video analyzing system in mice. J. Vis. Exp. 85, e50781, 10.3791/50871 (2014).
Acknowledgements
We thank T. Shiroshima, T. Kise, A. Nakakihara, M. Ishikawa and A. Imai for technical assistance and I. Yabe and E. Kushiya for help in generation of IL1RAPL1 knockout mice. This work was supported by Grant-in-Aid for Scientific Research (A) (KAKENHI Grant Number 24249014) and Grant-in-Aid for Scientific Research on Innovative Areas (KAKENHI Grant Numbers 22123008 and 221S0003) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
M.Y., T.Y., M.Y., K.T., K.S., T.K., T.M. and M.M. designed the study. M.Y., T.Y., M.Y., M.A., R.N., K.K. and T.U. performed experiments. M.Y., T.Y., K.K. and K.T. analysed the data. M.Y., T.Y., M.Y., K.K., T.K. and M.M. wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Yasumura, M., Yoshida, T., Yamazaki, M. et al. IL1RAPL1 knockout mice show spine density decrease, learning deficiency, hyperactivity and reduced anxiety-like behaviours. Sci Rep 4, 6613 (2014). https://doi.org/10.1038/srep06613
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06613
This article is cited by
-
Current Understanding of the Role of Neuronal Calcium Sensor 1 in Neurological Disorders
Molecular Neurobiology (2019)
-
Research of differential expression of sIL1RAP in low-grade gliomas between children and adults
Brain Tumor Pathology (2018)
-
The axon degeneration gene SARM1 is evolutionarily distinct from other TIR domain-containing proteins
Molecular Genetics and Genomics (2017)
-
Microglia contact induces synapse formation in developing somatosensory cortex
Nature Communications (2016)
-
AIM 2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.