Abstract
Electrical resistance is a material property that usually varies enormously with temperature. Constant electrical resistivity over large temperature range has been rarely measured in a single solid. Here we report the growth of Cu3NMx (M = Cu, Ag, Au) compound films by magnetron sputtering, aiming at obtaining single solids of nearly constant electrical resistance in some temperature ranges. The increasing interstitial doping of cubic Cu3N lattice by extra metal atoms induces the semiconductor-to-metal transition in all the three systems. Nearly constant electrical resistance over 200 K, from room temperature downward, was measured in some semimetallic Cu3NMx samples, resulting from opposite temperature dependence of carrier density and carrier mobility, as revealed by Hall measurement. Cu3NAgx samples have the best performance with regard to the range of both temperature and doping level wherein a nearly constant electrical resistance can be realized. This work can inspire the search of other materials of such a quality.
Similar content being viewed by others
Introduction
Electrical resistance is the most conventional and also the most useful property of a material. The temperature dependence of electrical resistance has to be taken into account when constructing devices and circuits. Remarkably, electrical resistance is a physical property that has an extremely large dynamical range of variation that for a specific material, often an electrical resistance variation by a few orders of magnitude can be measured1,2. It is just the easier manipulation of semiconductors' electrical resistance in a large dynamic range that lays the ground for our electronic era3. At the other extreme, for many an application electrical resistance that changes only slightly with temperature is also highly desirable. Such devices are usually realized with a few material components and/or in a compensative way, as in the alloy manganin which has to be prepared by a complicated series of heat-treatment. Considering the very large dynamic range of variation for electrical resistance, it would seem impracticable to demand a single solid to manifest a constant electrical resistance over a large temperature range. Yet the possibility of there existing single solids that display a nearly constant electrical resistance, and in a very large temperature range, over 200 K say, cannot be excluded. Intuitively, an indirect band semiconductor that can be modified to manifest semiconductor-to-metal transition arising from doping, by which the conduction band and valence band approach each other and may even cross over, is a good candidate, since when band cross-over occurs, the carrier density and carrier mobility can show an opposite tendency in response to temperature change.
Cu3N is a compound that has attracted much attention because of its potential application in various optical, electrical and magnetic devices4,5,6,7. Early work was focused on the optimal synthesis and its employment as write-once optical recording media4,8. In recently years, some other intriguing applications of Cu3N thin films or nanocrystals have also been explored, for example, to be used as electrode material in Li-ion batteries9, as cathode catalyst in alkaline fuel cells10, and as barrier layer in low resistance magnetic tunnel junctions11, to name just a few. The resistive-switching behavior in Cu3N thin films12, and the magnetic transition of 3d-metal doped Cu3N structures6 are also of great concern in recent research.
The stoichiometric Cu3N is an indirect-band semiconductor in the cubic anti-ReO3 type structure with a lattice constant of 0.383 nm (for the lattice structure, see Supplementary Fig. S1). This structure is rather open as the center of the primitive unit cell is vacant, thus it may accommodate another atom of Cu, Ag, Pd, Zn, etc. The interstitially doped Cu3N serves as a model system for the study of “enclosed atom”13,14. With the intercalation of more extra metal atoms in the host lattice, the material will experience a semiconductor-to-metal transition and will immediately turn into semimetallic in character13,15. When the doping level just rises over the critical value for transition corresponding to zero-gap, the material will show a very small temperature coefficient of resistance in some region of temperature, as can be conceived from the formula σ = neμe + nhμh, where n is the carrier density, and μ denotes the carrier mobility. This phenomenon was first confirmed by current authors in Cu3NPd0.2835. So far, the research about the interstitially doped Cu3N is dominated by theoretical investigations. First-principles calculation of the structural and electronic properties of Cu3NM compounds (with M = Ni, Cu, Zn, Pd, Ag and Cd) and some experiment results reveal that the incorporation of foreign metal atoms in the cell centers of the cubic lattice modifies the electronic structure of Cu3N and all such Cu3NM compounds are metallic13,14,15,16. Notably, report on the synthesis of the samples of well determined composition and atomic structure, and property measurement and mechanism (particularly for electrical transport) discussion based on experimental data are comparatively rare17,18. The difficulty lies in the growth of the intercalated samples with a well-preserved Cu3N host lattice. In fact, even the obtainment of a nearly stoichiometric Cu3N film is itself a challenge19.
In the current work we report the determination of temperature dependence of electrical resistance in the films of Cu3NMx (M = Cu, Ag, Au), here x denotes the fraction of the occupied cell centers in the Cu3N host lattice, which were grown by magnetron (co)sputtering of metal targets in the nitrogen atmosphere. The amount of extra metal atoms incorporated into the Cu3N lattice, denoted by the parameter x, was varied from zero to a sufficiently large value till the resulting material is essentially metallic. Samples of nearly constant electrical resistance, which was judged by the variance of the normalized electrical resistivity, in the range from room temperature down to ~ 50 K were obtained. The mechanism was discussed on the basis of measured carrier density and carrier mobility, together with the light absorption spectral data. These results can inspire further work on the search of single solids that will show constant electrical resistance in even larger temperature ranges, which promise more useful engineering applications.
Results
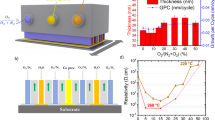
Thin films of nearly stoichiometric Cu3N as reference material and Cu3NMx (M = Cu, Ag, Au) of various compositions, as specified by x the fraction of the occupied cell centers in the Cu3N lattice, were grown. From x-ray diffraction (XRD) patterns it can be concluded that up to a quite large value of doping level, the host lattice of the Cu3N can be maintained, as confirmed by the characteristic (100)- and (200) reflections of Cu3N lattice (Fig. 1). When more Cu is interstitially doped into the deposits, the deposits, denoted as Cu3NCux, while maintaining the cubic structure of Cu3N, reveal an increasing lattice constant from a = 0.383 nm for Cu3N. From Fig. 1a one sees that, with x even going up to 0.70, the characteristic (100)- and (200) reflections on the XRD patterns remain well preserved while slightly shifting towards smaller angle. At x = 0.74, these two peaks disappear, and the (111) reflection emerges instead. At the same time, the lattice constant is dilated to a value of 0.385 nm. The film quality then becomes obviously degraded, i.e., less compact and less adhesive to the substrate, but such deposits are not of interest in the current work.
Similar behavior is expected to be observed in the Ag and Au doped-Cu3N structures. However, unlike in the case of Cu where the extra Cu atoms can be unambiguously attributed to the occupation of the cell centers of the Cu3N lattice, and the structure can be assuredly specified as Cu3NCux, whether the deposits obtained from co-sputtering of Cu with Ag (Au) can be specified as Cu3NAgx (Cu3NAux), i.e., whether the Ag (Au) atoms occupy uniquely the centers of the unit cells in the Cu3N host lattice, is a question that needs be clarified. Here we pointed out only that with the XRD patterns (Figs. 1b–1c) and other evidences, the deposits with Ag or Au here concerned can be definitely specified as Cu3NAgx or Cu3NAux (for more detailed argument, see supplementary information). In the following discussion such samples are referred to as Cu3NAgx or Cu3NAux, bearing in mind that in such materials the possibility of sporadic substitution of Cu atom on some sites by Ag (Au) atom cannot be completely excluded.
In the case of Ag doping, even though the (111) reflection of the Cu3N lattice already begins to be visible at x = 0.57, yet up to x = 0.85 the XRD patterns are still dominated by the (100)- and (200) reflections (Fig. 1b). The lattice constant increases monotonically from 0.383 nm for Cu3N to 0.398 nm for Cu3NAg. In the case of Au doping, since the Au has a larger ionic radius, the insertion of Au atoms causes severe dilation of the Cu3N lattice that the shift of the (100)- and (200) reflections is quite obvious (Fig. 1c). We obtained high-quality Cu3NAux films with x up to 0.49, all showing well preserved (100)- and (200) reflections characterizing the Cu3N lattice. In the given range of Au doping, the semiconductor-to-metal transition of interest already occurs in the deposits. The dependence of lattice constant on the fraction of occupied cell centers for the three sample series is summarized in Fig. 1d.
For the nearly stoichiometric Cu3N sample, its electrical resistance exhibits an exponential growth with decreasing temperature (see Supplementary Fig. S2a), which is typical for an intrinsic semiconductor in which the electronic transport is dominated by thermal activation. This temperature dependence of electrical resistance can be approximated by the equation ρ(T) = C exp(−Ea/kT), with the activation energy Ea ~ 0.2 eV. This Ea deviates a lot from the Eg/2 in the formula for a perfect intrinsic semiconductor Cu3N which should have a band gap of ~ 1.9 eV20. This weird behavior results from the fact that even the stoichiometric Cu3N sample is composed of nanocrystallites, generally of ~ 40 nm in size, enclosed by Cu-terminated {111}-planes which provide alternative hopping paths for charge carriers18. Largely due to the same reason, Cu3N is also notoriously known for the vast discrepancy in the reported band gap values. With the incorporation of extra Cu, or foreign atoms Ag or Au, the electrical resistance at room temperature of the samples decreases steadily with increasing x in Cu3NMx (M = Cu, Ag, Au) (Fig. 2a), indicating the drastic change in the band structure induced by the interposition of extra atoms into the Cu3N lattice. The temperature dependence of electrical resistivity for some samples of the Cu3NMx (M = Cu, Ag, Au) series is displayed in Figs. S2–S4, see the Supplementary information.
With increasing extra Cu in the deposit, the deposit Cu3NCux reveals a steadily reduced electrical resistance in the temperature range from room temperature down to 5 K. Moreover, for x = 0.59 onward, with decreasing temperature the electrical resistance initially decreases to a minimum and then rises again. In fact, with further increasing x this minimum shifts steadily towards lower temperature. For the sample Cu3NCu0.74, the curve reveals typical behavior of a metal, which falls in the regime of conventional Fermi liquid theory2, and the minimum appears at ~ 40 K (see Supplementary Fig. S2 & Fig. 2b). This confirms the occurrence of semiconductor- to-metal transition in Cu3NCux with the doping level in the neighborhood of x = 0.59.
It is anticipated that at the point of the semiconductor-to-metal transition, in fact when the material is slightly more at the metal side, thus the indirect-band semiconductor has turned into a semimetal13,14, the electrical resistance may be less sensitive to temperature change, as the opposite tendency of variation for carrier density and carrier mobility in this situation may compensate each other to some extent. Fig. 2b shows the temperature dependence of electrical resistance normalized against the values at room temperature for samples Cu3NCux with x ranging from 0.36 to 0.74 (noting that the electrical resistance at room temperature decreases to 2.3 × 10−6 Ω·m, see Fig. 2a). It can be seen that for the sample Cu3NCu0.60, in the temperature range from room temperature down to 40 K, i.e., within a temperature range of 260 K, the normalized electrical resistance varies within the range of ± 0.002, and the variance, defined as  , where
, where  is the averaged value of the normalized electrical resistance, is only 8.6 × 10−4.
is the averaged value of the normalized electrical resistance, is only 8.6 × 10−4.
Fig. 2c displays the temperature dependence of the normalized electrical resistance for samples Cu3NAgx with x ranging from 0.41 to 1.0 (At the same time, the electrical resistance at room temperature decreases to 3.0 × 10−6 Ω·m for Cu3NAg). Remarkably, the electrical transport behavior for the sample x ~ 0.85 is typically metallic. For the sample with x ~ 0.76 which is slightly larger than x ~ 0.73 where the semiconductor-to-metal transition just occurs, the normalized electrical resistance varies within ± 0.002 in the temperature range from room temperature down to 55 K, and the variance of the normalized electrical resistance in this temperature range as large as 245 K is only 8.0 × 10−4.
In comparison to cases of Cu and Ag insertion, the semiconductor-to-metal transition occurs already at x ~ 0.33 in the Cu3NAux system (Fig. 2d). A very flat variation of electrical resistance with temperature in a range as wide as 100 K was not measured. In the sample Cu3NAu0.37, the variance of the normalized electrical resistance in the range from room temperature to 150 K measures 3.3 × 10−4. The scenario that the normalized electrical resistance varies within ± 0.002 in a temperature range over 200 K was not measured in this system.
By comparing the temperature dependence of the normalized electrical resistivity for the three sample series, it can be said that the Cu3NAgx structure makes the best performance to provide nearly constant electrical resistivity in wide temperature range, considering both the smallness of variance of the electrical resistivity and the range of temperature within which the measurement was made.
Discussion
The temperature dependence of the electrical resistivity confirms that the interstitial doping of the Cu3N lattice turns Cu3NMx (M = Cu, Ag, Au) gradually to be metallic when x increases over a critical value. As speculated by theoretical simulation, in this process the intercalated structures will show a narrowing band gap, which can be revealed by the optical absorption spectrum. Taking the series of Cu3NAgx for instance, the samples with x up to 0.57 shows perfect linearity in the (αhν)1/2 vs. hν curves, pointing towards a continuously narrowing band-gap (see Supplementary Fig. S5). At x = 0.73, the deposit has a zero band-gap, and since then on the deposits become metallic. At the doping level slightly larger than the critical value for the transition, the conduction band and the valence band may become overlapped.
The occupation of the cell centers of the Cu3N lattice by extra Au, Ag, Cu atoms, when the doping is not so heavy, turns the derived structures into a n-type semiconductor, as confirmed by the negative Hall coefficient. Since in the Cu3NMx compouds the conduction, albeit ambipolar in fact, is dominated by electrons provided by excessive metal atoms, we can assume that neμe is considerably larger than nhμh so as to roughly estimate the electron density and mobility from the measured electrical resistivity and Hall coefficient. As anticipated, with the insertion of more extra Au, Ag, and Cu atoms, the carrier density at room temperature increases monotonically. Taking Cu3NAgx as example, the carrier density jumps from 7.0 × 1017/cm3 at x = 0.0 to 8.6 × 1021/cm3 when x approaches 1.0. In contrast, the carrier mobility shows a minimum at x ~ 0.73 where the structure may show a maximum disorder due to the insertion of extra metal atoms into the host lattice (Fig. 3a). The quantitative detail is difficult to be specified since the mobility depends strongly on the quality of the individual samples. In Cu3NCux and Cu3NAux where the x value is not very large, the mobility decreases monotonically with x in the given range. By the way, it is remarkable that samples such as Cu3NAgx with large x value have a carrier density comparable with metal, albeit they show a temperature dependence of electrical resistivity typical for semiconductor. The reason lies in the fact that in conventional semiconductors such as n-type Si, the carrier is provided by ionized impurity atoms occupying lattice sites, of which the density is consequently rarely higher than 1021/cm3. However, in Cu3NMx it is the excessive metal atoms, the electrons can freely dissociate from such parent atoms, that occupy the originally vacant cell centers of the cubic lattice, which modify the band structure towards a narrower gap till eventually becoming fully metallic13, and at the same time contribute a large amount of carriers.
From Fig. 3b it can be seen that in a particular doped specimens, Cu3NAg0.57 for instance, the carrier density becomes less with decreasing temperature, while the carrier mobility shows a reversed tendency, a favorable condition for having a slowly varying product of carrier density and carrier mobility. For the sample Cu3NAg0.73, although the data become more scattering, the same tendency is observed, and for both carrier density and carrier mobility the amplitudes of variation in the given temperature range become abated, which then result in a only slightly varied product, as also confirmed by the temperature variation of the electrical resistance (Cf. Fig. 2c).
That the product of carrier density and carrier mobility from the Hall measurement (Figs. 3b–3d) is not consistent with the electrical resistance is due to the fact that the electrical conductivity is contributed by both electrons and holes, whereas the contribution of the minor carrier (here the hole) to the Hall measurement is not accounted for in the same way as to the electrical resistance. The scattering of carriers by phonons of an imperfect lattice, in which the center of the cubic unit cells is randomly and partially occupied, is believed to be the controlling mechanism for electrical resistivity. For the deposits here concerned, which comprise of Cu-enclothed nanocrystallites, the “variable range hopping” among the nanocrystallites21, i.e., the charges' quantum tunneling between localized sites, is also a governing mechanism that may also demonstrate its effect in the temperature dependence of electrical resistance. Anyway, in spite of these disturbing factors that contribute to the electrical resistance, it can be confirmed that at the neighborhood of transition in the Cu3NCux and Cu3NAgx, a nearly constant resistance is obtainable in a large temperature range over 200 K. The detailed quantitative investigation into the mechanism can be better realized in materials having a better crystallinity, which is now strived for in our laboratory.
In summary, thin films of the compounds Cu3NCux, Cu3NAgx and Cu3NAux were synthesized by magnetron sputtering of metal targets in nitrogen atmosphere. The increasing fraction of occupied cell centers in the host Cu3N lattice by extra Au, Ag or Cu atoms induces the semiconductor-to-metal transition in these solids. At the metallic side not far from the semiconductor-to-metal transition, the carrier density in the samples increases with the increasing temperature due to the thermal excitation, while the carrier mobility declines due to the more violent lattice scattering among others. This brings about the possibility of obtaining some single solids of nearly constant electrical resistance over a large temperature range, which has been observed in all the Cu3NCux, Cu3NAgx and Cu3NAux systems. Remarkably, the sample Cu3NAg0.76, the variance of the normalized electrical resistivity, in a temperature range from room temperature to 55 K, is only ~ 8.0 × 10−4. As for the transport mechanisms, thermal activation and variable hoping range dominate the semiconductor state, while Fermi liquid theory can account for the metal state. The current research has verified the possibility of obtaining nearly constant electrical resistivity in single solids, and offers clues of finding more such materials in even larger temperature ranges, and better if extended farther above the room temperature. Single solids of such a quality are highly desirable in the fabrication of precision resistors and other devices based on them that are to be used in environment of variable temperatures.
Methods
Film preparation
Cu3NMx (M = Cu, Ag, Au) films were prepared onto c-Si and quartz substrates in a custom-designed radio-frequency (27.12 MHz) reactive magnetron sputtering system, with high purity (5 N) nitrogen as the working gas. In order to obtain Cu3NAgx and Cu3NAux samples of various doping levels, chips of Au or Ag metal in a size of 2 × 2 mm were placed in a symmetrical way onto the ring-formed sputter region of the Cu target. The content of Ag or Au in the ternary deposits can be adjusted by changing the number of the metal chips on the Cu target and/or slightly varying their positions with regard to the sputtered region, while for the case of Cu doping this was realized via adjusting the ratio of nitrogen versus argon in the working gas. Before mounting into the chamber, the substrates were ultrasonically cleaned in acetone, alcohol and deionized water, successively. The working parameters are as follows: The target-substrate distance was 50 mm, the working pressure in the chamber was kept at 1.3 Pa, and the RF power was tuned to 60 W. The procedure of obtaining nearly stoichiometric Cu3N samples and control of sample composition was described in our previous publications18,19.
Characterization
The surface morphology of the as-deposited films was evaluated by using a scanning electron microscope (XL30 S-FEG), and the film thickness was determined by a Talystep profilometer (Veeco Dektak 8). The crystal structure of the as-deposited films was characterized by using X-ray diffraction (XRD, Ultima IV, Rigaku) with the Cu Kα line. More information concerning the precise determination of the lattice constant in the deposits was obtained by using a high-resolution transmission electron microscope (TEM, JEOL 2010). X-ray photoelectron spectroscopy (XPS, ESCALAB250Xi, Thermo Scientific) measurement was used to determine the chemical bonding states and composition of the deposits. The sample composition determination was done with the nearly stoichiometric binary Cu3N as reference. Temperature dependence of electrical resistance and Hall effect under a magnetic field of 5 Tesla were measured in the temperature range from room temperature down to 5 K by employing the five-contact method in a physical property measurement system (PPMS 6000, Quantum Design). The resistivity was determined by using the conventional four-point probe collinear technique, while the Hall measurement was made in a geometry with the four contacts on the corners of a square. The optical gap of the deposits was evaluated on the basis of the absorption data recorded by a UV-Vis spectroscopy (SP2500i, Princeton instruments). For the PPMS measurement, thinner films (~60 nm in thickness) were used, while for XRD characterization the samples are generally as thick as ~ 1.0 μm.
References
Hummel, R. E. Electronic Properties of Materials (Springer, New York, 2001).
Ashcroft, N. W. & Mermin, N. D. Solid State Physics (Thomson Learning Inc., 1976).
Yu, P. Y. & Cardona, M. Fundamentals of Semiconductors (Springer, New York, 2001).
Maruyama, T. & Morishita, T. Copper nitride and tin nitride thin films for write-once optical recording media. Appl. Phys. Lett. 69, 890–891 (1996).
Ji, A. L., Li, C. R. & Cao, Z. X. Ternary Cu3NPdx exhibiting invariant electrical resistivity over 200 K. Appl. Phys. Lett. 89, 252120 (2006).
Cui, X. Y. et al. First principles study of 3d transition metal doped Cu3N. J. Magn. Magn. Mater. 324, 3138–3143 (2012).
Yue, G. et al. Copper nitride thin film prepared by reactive radio-frequency magnetron sputtering. J. Appl. Phys. 98, 103506 (2005).
Nosaka, T., Yoshitake, M., Okamoto, A., Ogawa, S. & Nakayama, Y. Thermal decomposition of copper nitride thin films and dots formation by electron beam writing. Appl. Surf. Sci. 169, 358–361 (2001).
Pereira, N., Dupont, L., Tarascon, J. M., Klein, L. C. & Amatucci, G. G. Electrochemistry of Cu3N with lithium a complex system with parallel processes. J. Electrochem. Soc. 150, A1273–A1280 (2003).
Wu, H. & Chen, W. Copper nitride nanocubes: size-controlled synthesis and application as cathode catalyst in alkaline fuel cells. J. Amer. Chem. Soc. 133, 15236–15239 (2011).
Borsa, D. M., Grachev, S., Presura, C. & Boerma, D. O. Growth and properties of Cu3N films and Cu3N/γ-Fe4N bilayers. Appl. Phys. Lett. 80, 1823–1825 (2002).
Zhu, W. et al. Resistive-switching behavior and mechanism in copper-nitride thin films prepared by DC magnetron sputtering. Phys. Status Solidi A 209, 1996–2001 (2012).
Moreno-Armenta, M. G., Perez, W. L. & Takeuchi, N. First-principles calculations of structural and electronic properties of Cu3MN compounds with M = Ni, Cu, Zn, Pd, Ag, and Cd. Solid State Sci. 9, 166–172 (2007).
Moreno-Armenta, M. G., Martínez-Ruiz, A. & Takeuchi, N. Ab initio total energy calculations of copper nitride: the effect of lattice parameters and Cu content in the electronic properties. Solid State Sci. 6, 9–14 (2004).
Hahn, U. & Weber, W. Electronic structure and chemical-bonding mechanism of Cu3N, Cu3NPd, and related Cu(I) compounds. Phys. Rev. B 53, 12684–12693 (1996).
Pierson, J. F. & Horwat, D. Addition of silver in copper nitride thin films deposited by reactive magnetron sputtering. Scripta Mater. 58, 568–570 (2008).
Gao, L., Ji, A. L., Zhang, W. B. & Cao, Z. X. Insertion of Zn atoms into Cu3N lattice: structure distortion and modification of electronic properties. J. Cryst. Growth 321, 157–161 (2011).
Ji, A. L., Gao, L., Du, Y. & Cao, Z. X. Crystalline thin films of stoichiometic Cu3N and intercalated Cu3NMx (M = metals): growth and physical properties. Phys. Status Solidi A 207, 2769–2780 (2010).
Ji, A. L., Huang, R., Du, Y., Li, C. R. & Cao, Z. X. Growth of stoichiometric Cu3N thin films by reactive magnetron sputtering. J. Cryst. Growth 295, 79–83 (2006).
Du, Y., Ji, A. L., Ma, L. B., Wang, Y. Q. & Cao, Z. X. Electical conductivity and photoreflectance of substoichiometric copper nitride thin films deposited at low temperature. J. Cryst. Growth 280, 490–494 (2005).
Mott, N. F. & Davis, E. A. Electronic Processes in Non-crystalline Materials (Clarendon Press, Oxford, 1979).
Acknowledgements
This work was financially supported by the Innovation Program of the Chinese Academy of Sciences, by the National Natural Science Foundation of China Grant nos.11290161, 51172272 and 10904165, and by the National Basic Research Program of China grant nos. 2009CB930801 and 2012CB933002.
Author information
Authors and Affiliations
Contributions
Z.X.C. and A.L.J. initiated the current research; N.P.L. performed the experiment; Z.X.C. compiled the manuscript; all the authors contributed to analysis of the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Nearly Constant Electrical Resistance over Large Temperature Range in Cu3NMx (M = Cu, Ag, Au) Compounds (PDF 450 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lu, N., Ji, A. & Cao, Z. Nearly Constant Electrical Resistance over Large Temperature Range in Cu3NMx (M = Cu, Ag, Au) Compounds. Sci Rep 3, 3090 (2013). https://doi.org/10.1038/srep03090
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03090
This article is cited by
-
Copper nitride/silver nanostructures synthesized via wet chemical reduction method for the oxygen reduction reaction
Journal of Nanoparticle Research (2023)
-
Effect of Magnetic Transition Metal (TM = V, Cr, and Mn) Dopant on Characteristics of Copper Nitride
Journal of Superconductivity and Novel Magnetism (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.